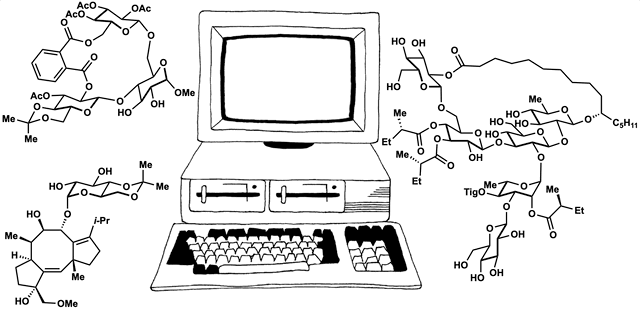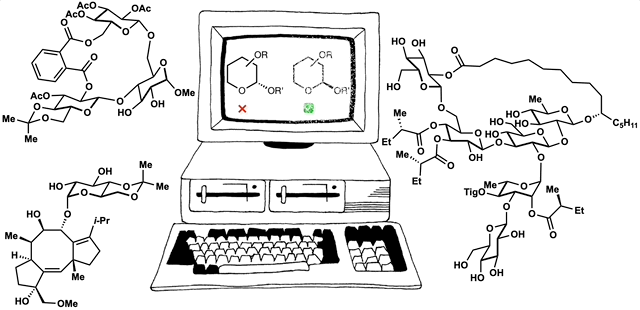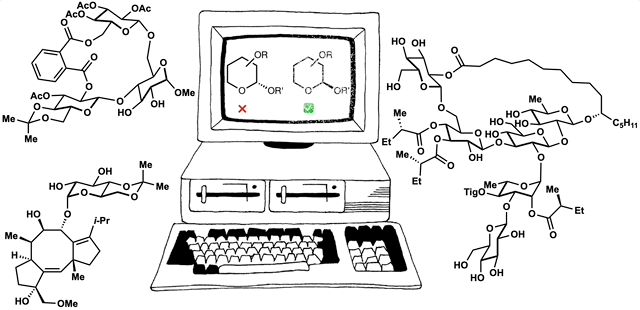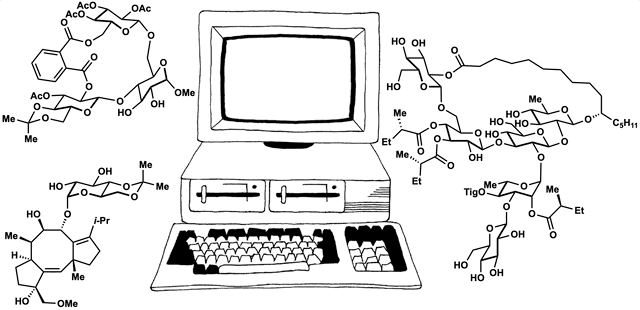
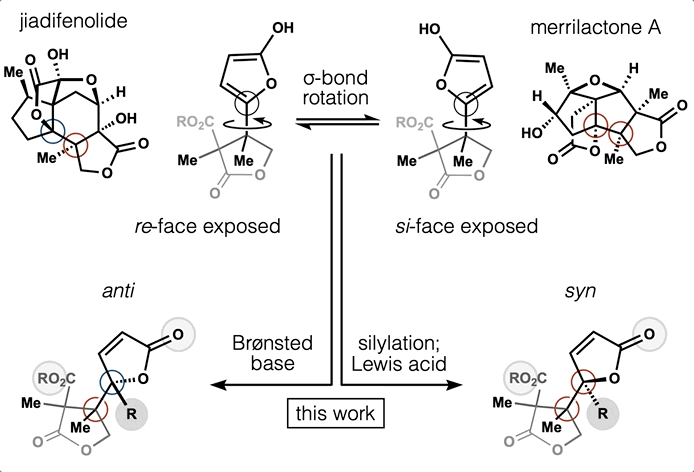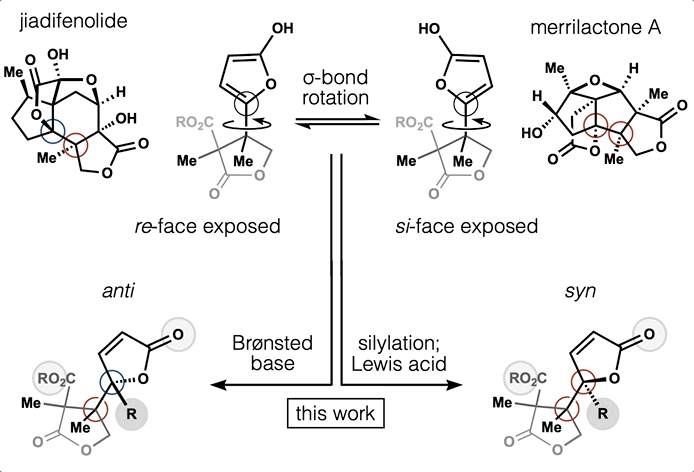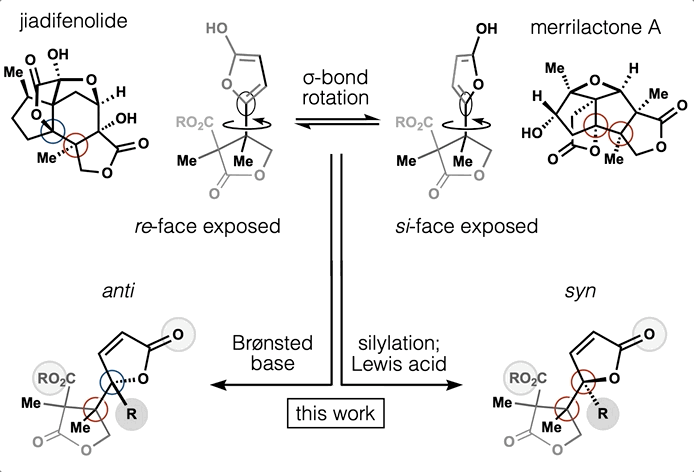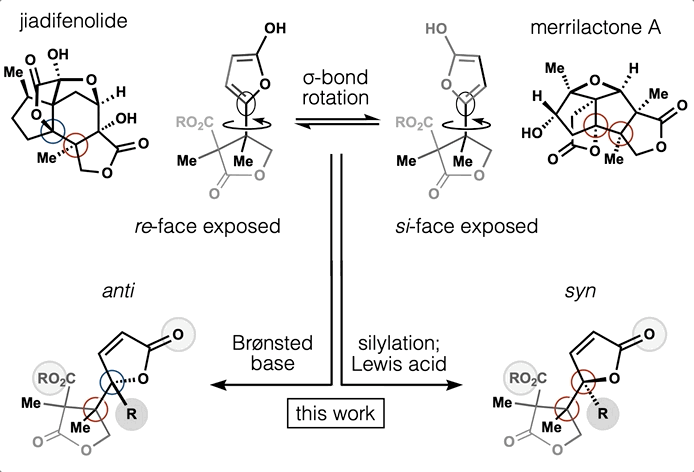
Computed Affinity / Dynamically Ordered Retrosynthesis
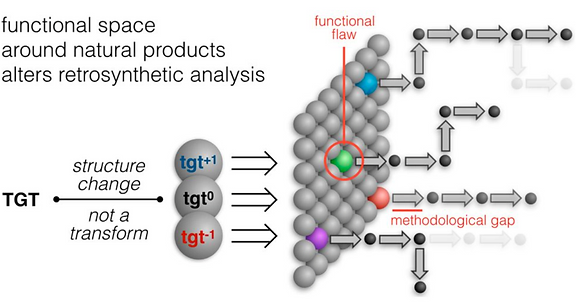
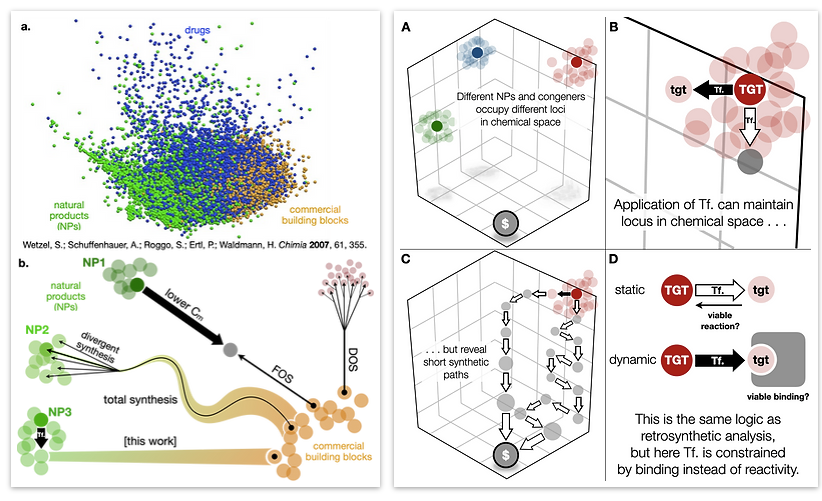
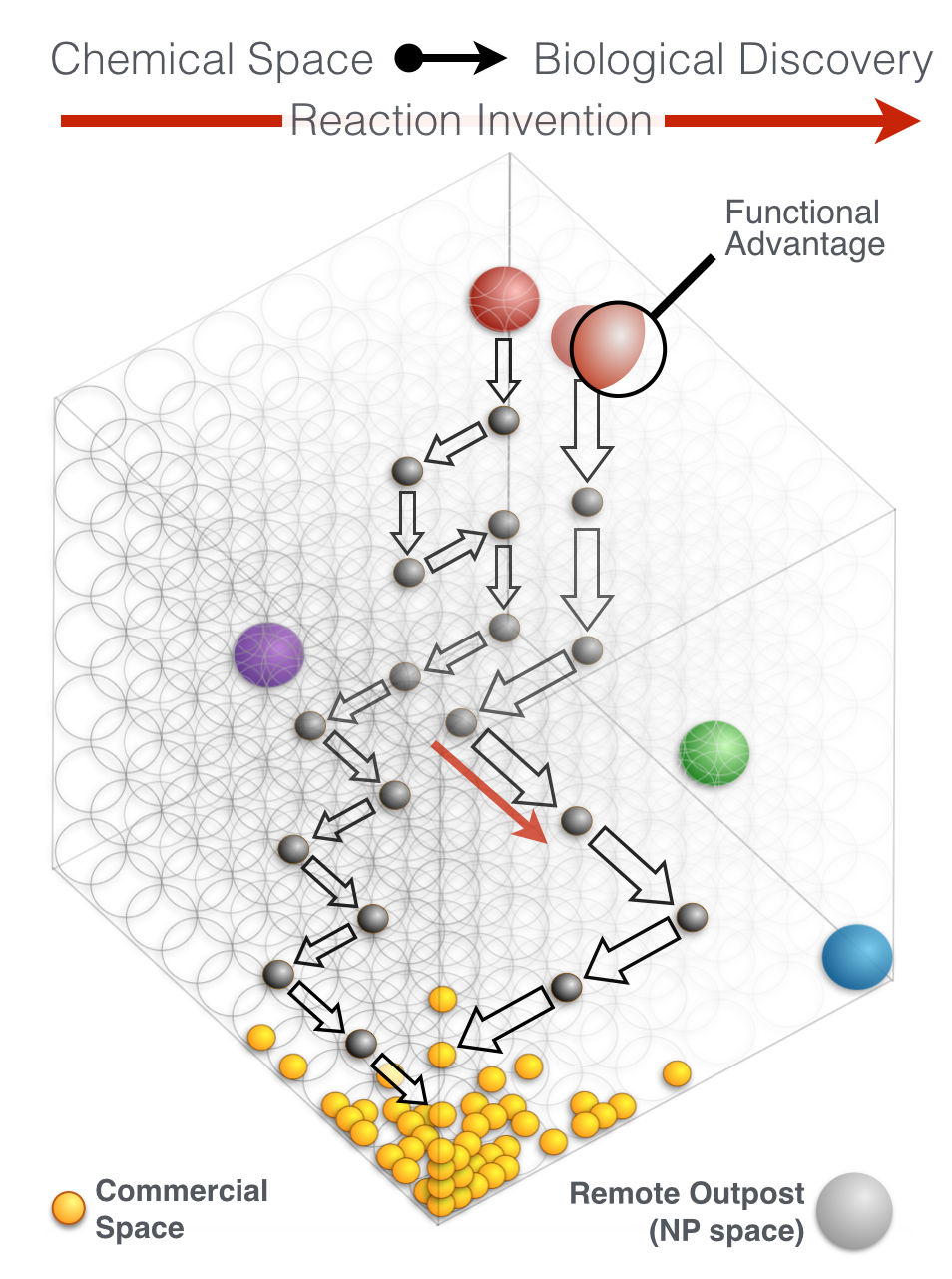
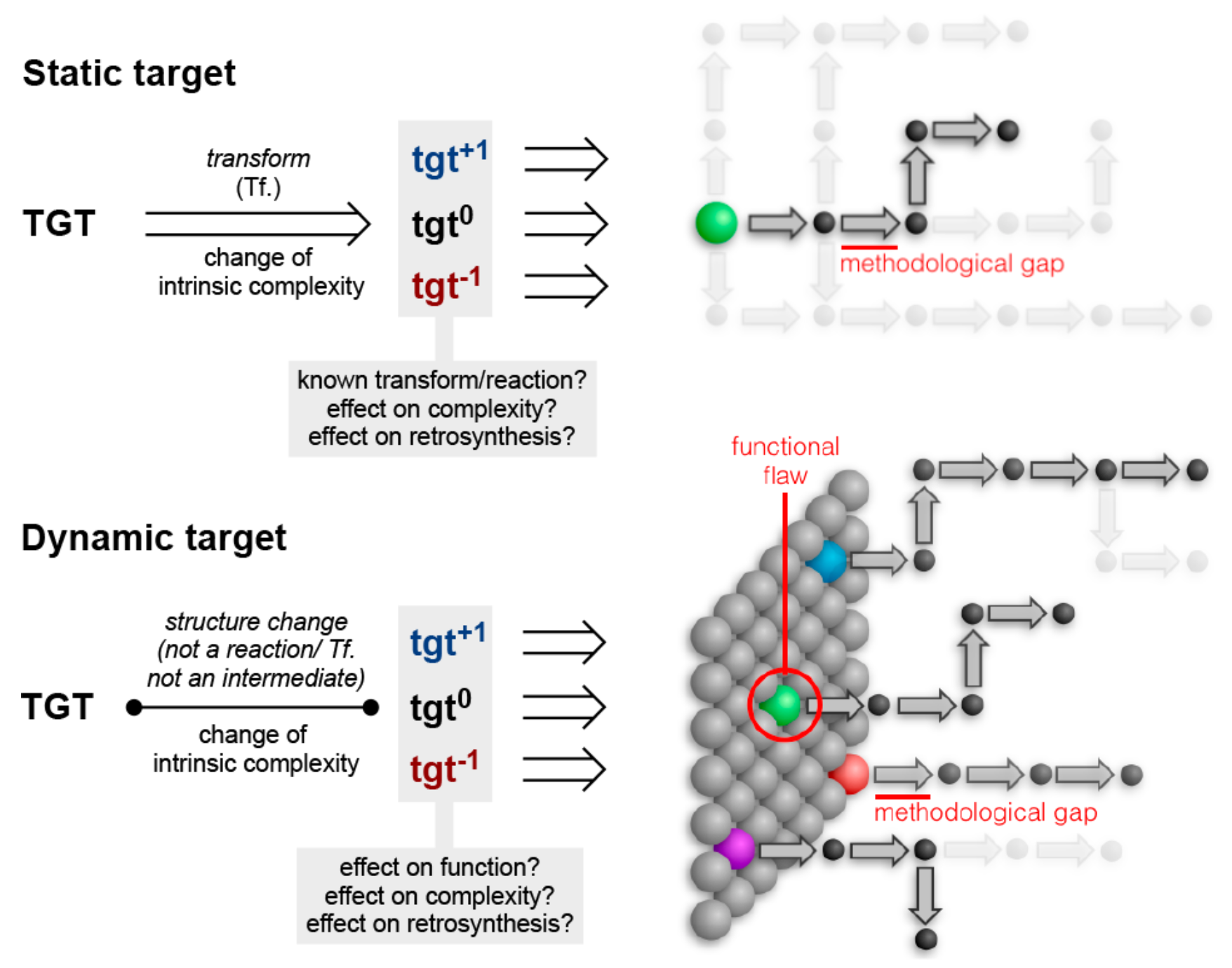
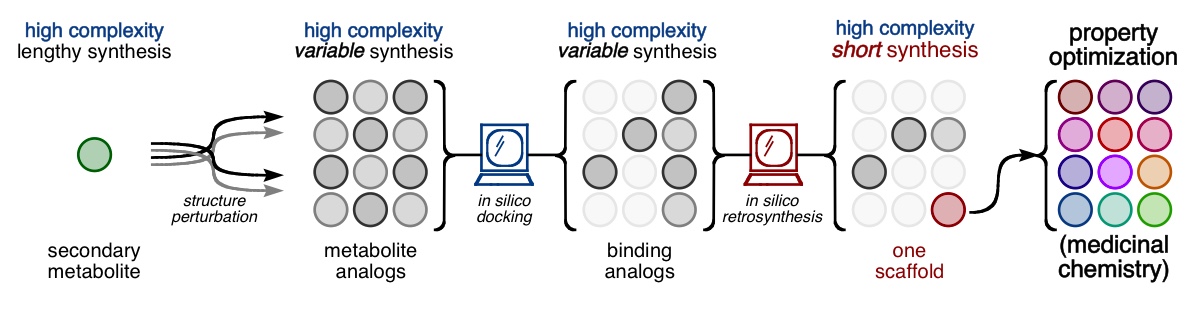
Every molecule extends numerous retrosynthetic paths. But different molecules, even molecules that differ by a single atom, will possess different retrosyntheses: some longer, some shorter. Therefore small alterations of a complex molecule may reveal a shorter synthesis than is possible for the native molecule itself. This structural perturbation with retrosynthetic analysis we will define as ‘dynamic retrosynthetic analysis’ since it follows exactly the same thought process as retrosynthetic analysis itself, where the TGT is static. Structural changes to the TGT will increase, maintain, or decrease structural complexity, which affects downstream transforms to reach starting materials. The key difference is that these initial perturbations to the TGT can include nonsense transforms that need not correspond to possible reactions. This logic allows changes to natural products that retain their structural complexity but reduce synthetic burden. In contrast to our major inspiration for this approach—Wender’s Function-Oriented Synthesis (FOS)—structural complexity and synthetic complexity (# steps) are not linearly correlated. Structural and synthetic complexity are, instead, distinct. (to calculate molecular complexity using a SMILES string, try the tool developed by Forli Lab)
The value of this approach is in deployment of natural products against biological targets. A validated target can also narrow the field of possible analogs by in silico docking. This workflow (see below) is now being investigated as a general tool for synthetic chemistry.
89. Fernandez, S. A.; Snelson, D. W.; Shenvi, R. A. “Digital Duet: Synergism between computation and the synthesis of glycosides” TCI Mail, 2025, accepted.
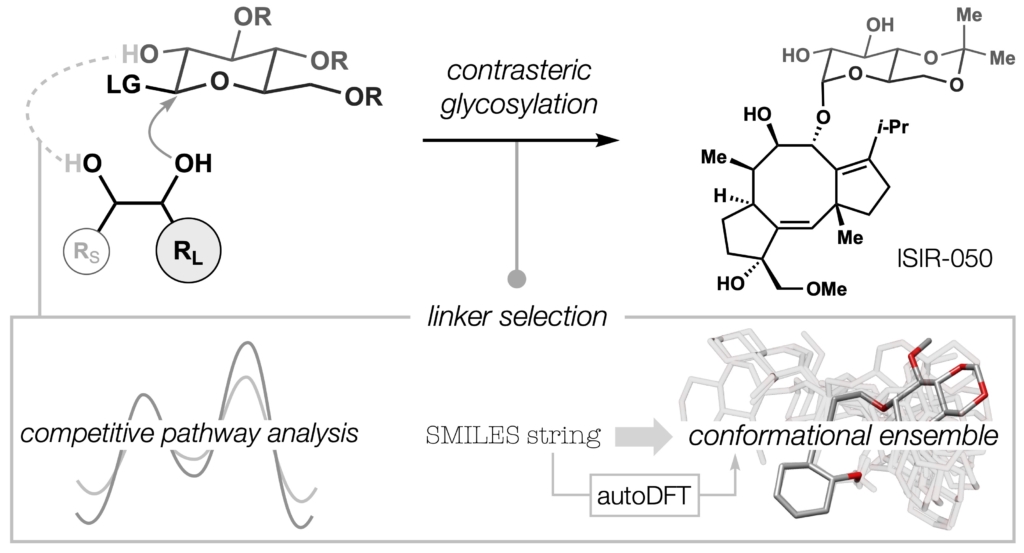
86. Snelson, D.;† Ting, S.;† Shenvi, R. A. “Contrasteric glycosylations of cotylenol and 1,2-diols by virtual linker selection” J. Am. Chem. Soc. 2025, 147, 1327–1333.
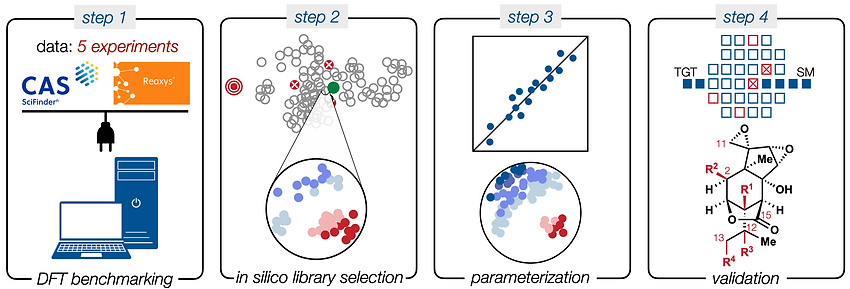
85. Li, C.; Shenvi, R. A. “Total synthesis of twenty-five picrotoxanes by virtual library selection” Nature, 2024, accepted. ChemRxiv DOI: 10.26434/chemrxiv-2024-g8j1r
79. Shenvi, R. A. “Natural product synthesis in the 21st century: beyond the mountain top” [invited Perspective] ACS Cent. Sci. 2024, 10, 519–528.

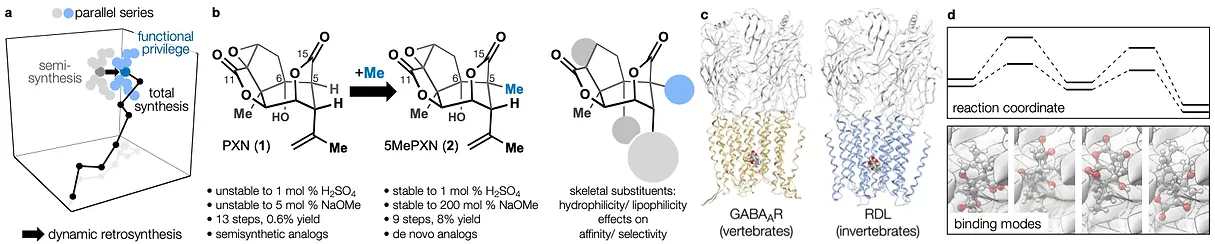
78. Tong, G.; Griffin, S.; Sader, A.; Crowell, A. B.; Beavers, K.; Watson, J.; Buchan, Z.; Chen, S.; Shenvi, R. A. C5 Methylation Confers Accessibility, Stability and Selectivity to Picrotoxinin Nature Commun. 2023, 14, 8308.
ChemRxiv DOI: 10.26434/chemrxiv-2022-0l4nt
72. Hill, S. J.§; Dao, N.§; Dang, V.; Stahl, E.; Bohn, L. Shenvi, R. A. A route to potent, selective and biased salvinorin chemical space. ACS Cent. Sci., 2023, online. §co-first authors
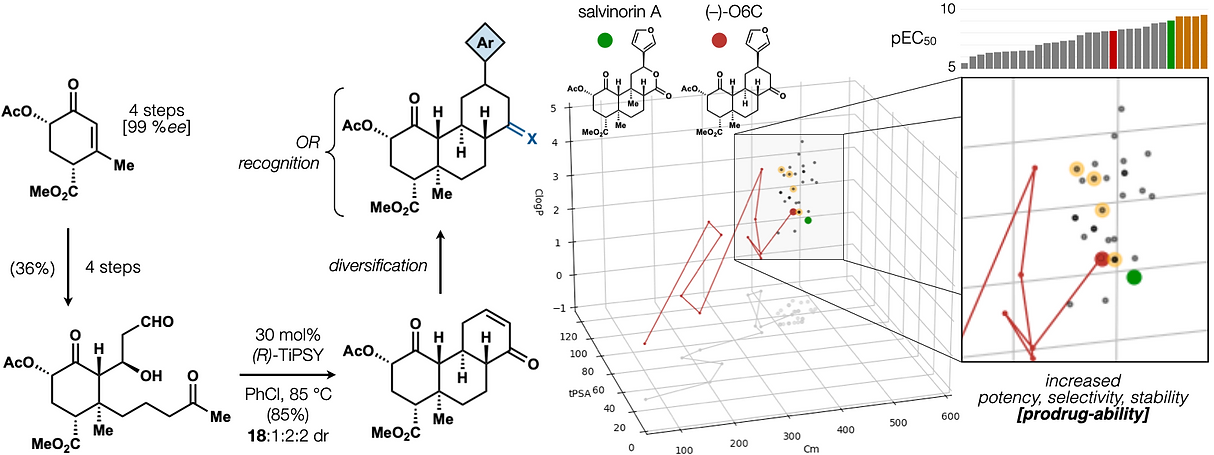
62. Woo, S.; Shenvi, R. A. Natural Product Synthesis through the Lens of Informatics Acc. Chem. Res. 2021, 54, 1157–1167.
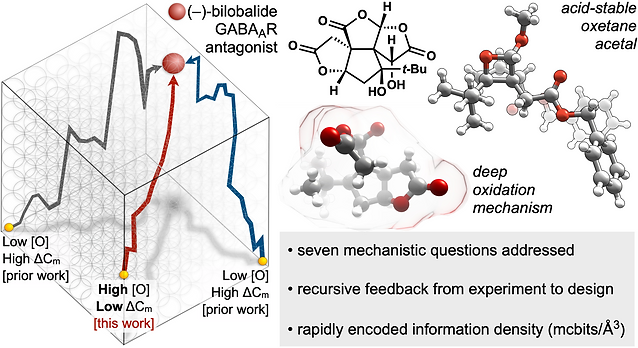
59. Demoret, R. M.; Baker, M. A.; Ohtawa, M.; Chen, S.; Lam, C.-C.; Khom, S.; Roberto, M.; Forli, S.; Houk, K.; Shenvi, R. A. Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide. J. Am. Chem. Soc. 2020, 142, 18599–18618.ChemRxiv DOI: 10.26434/ chemrxiv.12132939.v2
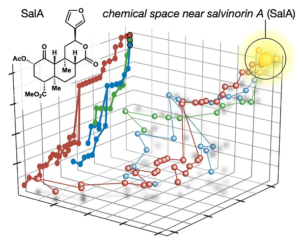
58. Hill, S. J.; Brion, A. U. C. M.; Shenvi, R. A. Chemical Syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020, 37, 1478–1496.

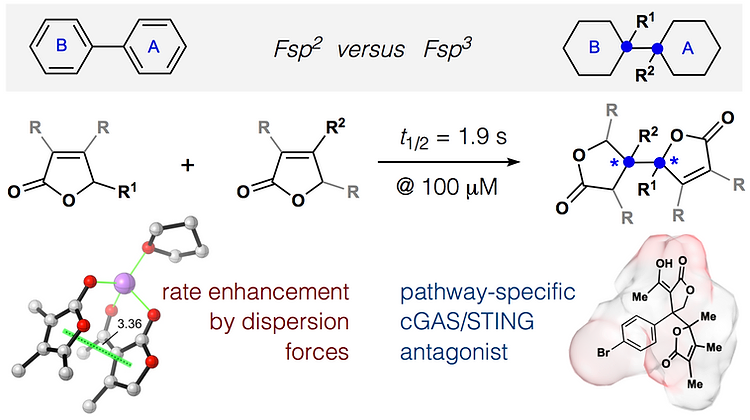
54. Huffman, B. J.; Chen, S.; Schwarz, J. L; Plata, R. E.; Chin, E.; Houk, K. N.; Lairson, L. L.; Shenvi, R. A. Electronic Complementarity Permits Hindered Butenolide Heterodimerization and Discovery of Novel cGAS/STING Pathway Antagonists, Nature Chem. 2020, 12, 310–317.
48. Huffman, B.; Shenvi, R.A. Natural Products in the ‘Marketplace’: Interfacing Synthesis and Biology J. Am. Chem. Soc. 2019, 141, 3332-3346.