2025
90. Freeman, S. M.; Landwehr, E. M.; Rojas, J. R.; Tanaka, R.; Bailey, J. B.; Gembicky, M.; Shenvi, R. A.* “Decagram-scale synthesis of GB13, a Galbulimima alkaloid precursor” 2025, ChemRxiv doi: 10.26434/chemrxiv-2025-490r9

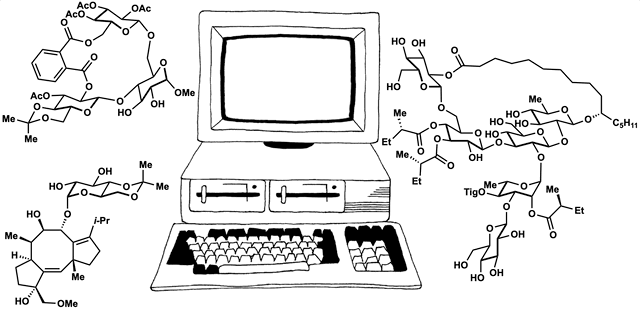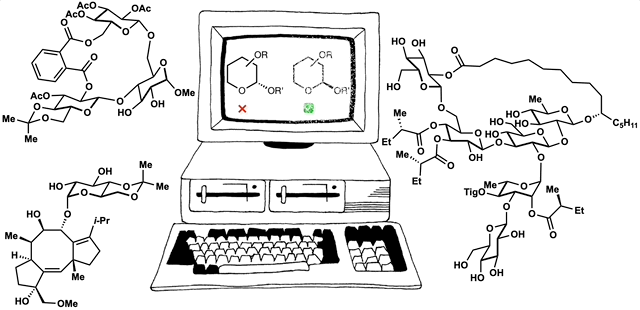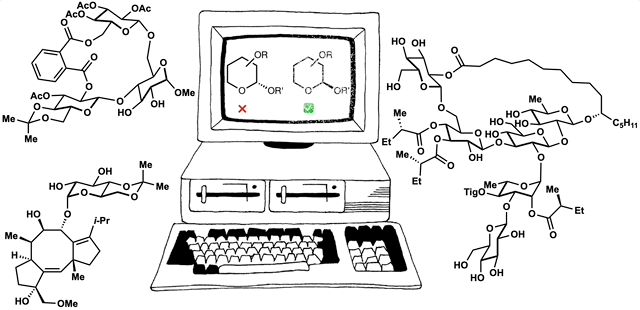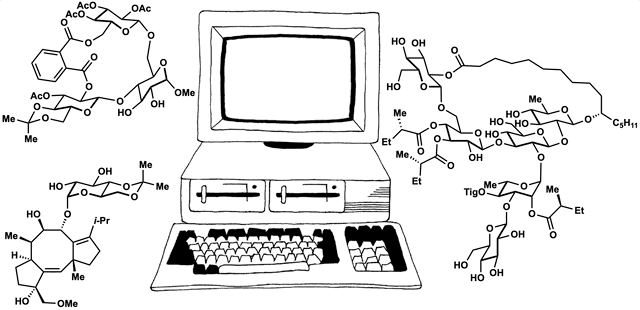
89. Fernandez, S. A.; Snelson, D. W.; Shenvi, R. A. “Digital Duet: Synergism between computation and the synthesis of glycosides” TCI Mail, 2025, accepted.
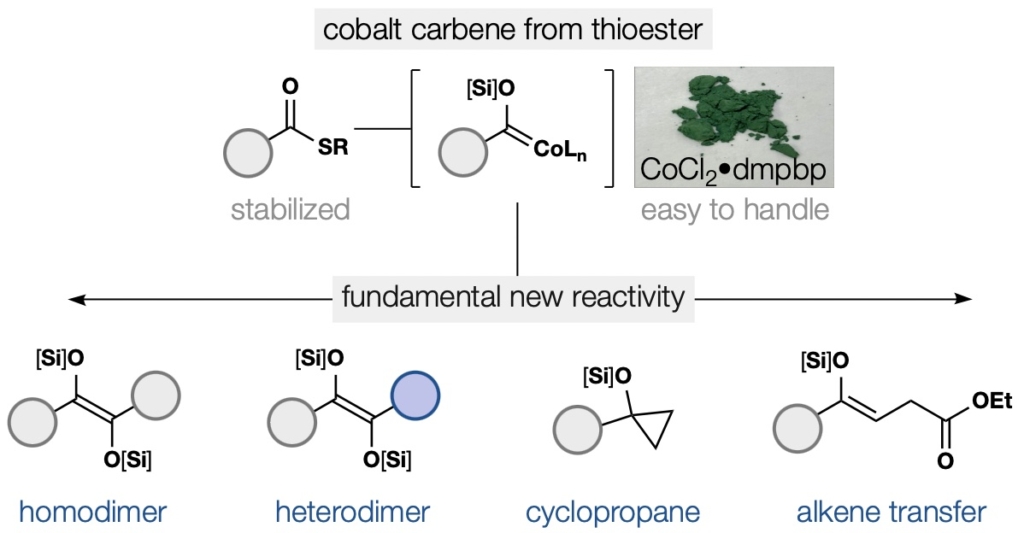
88. Kong, L.;‡ Zong, K.;‡ Guo, J.; Shenvi. R. “Catalytic Acyloin-Type Heterocoupling of Thioesters via a Putative Cobalt Siloxycarbene” 2025, submitted, ChemRxiv doi: 10.26434/chemrxiv-2025-hxpsz
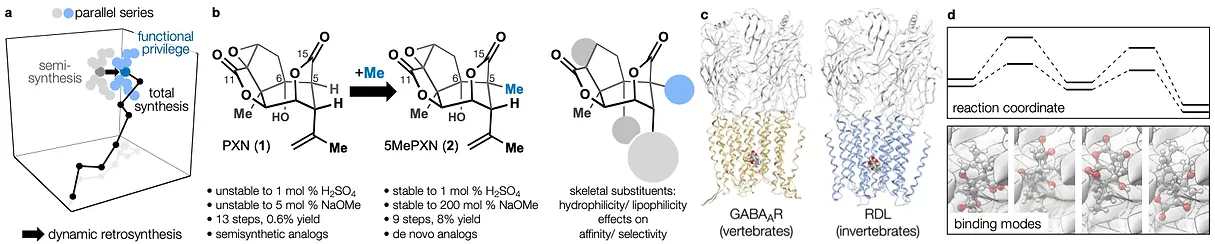
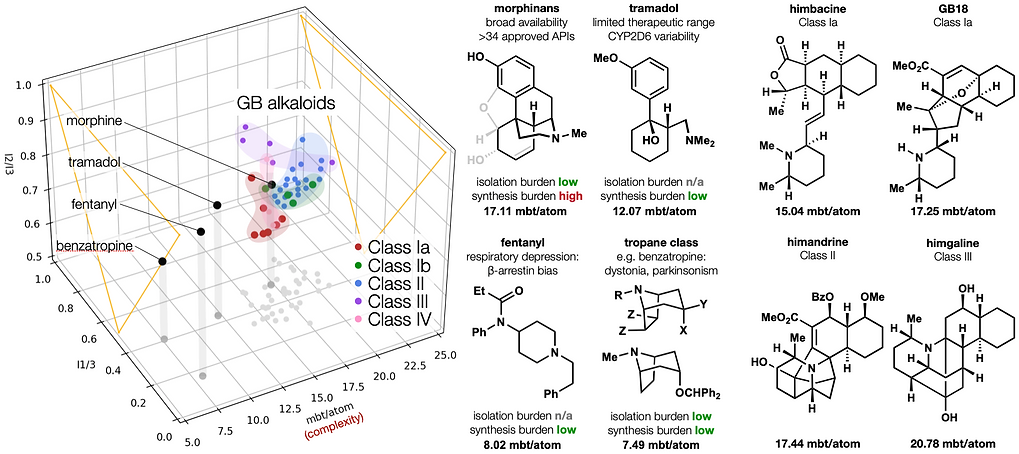
87. Zielke, F.;† Woo, S.;† Kasmali, S.; Volf, A.; Dang, V.; Bailey, J. B.; Gembicky, M.; Bohn, L. M.;* Shenvi. R.* “Bidirectional modification of a Galbulimima alkaloid identifies selective opioid ligands” ACS Central Science, 2025, online. ChemRxiv DOI: 10.26434/chemrxiv-2025-642tw.

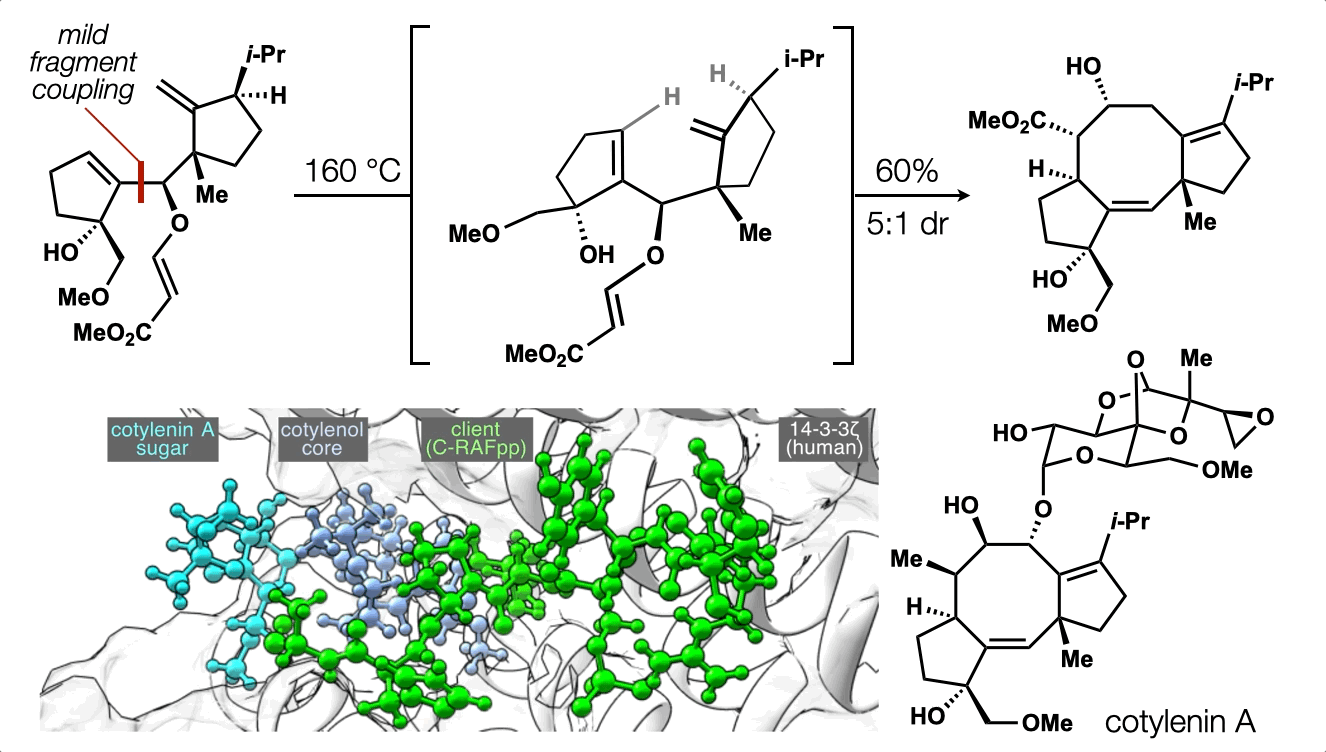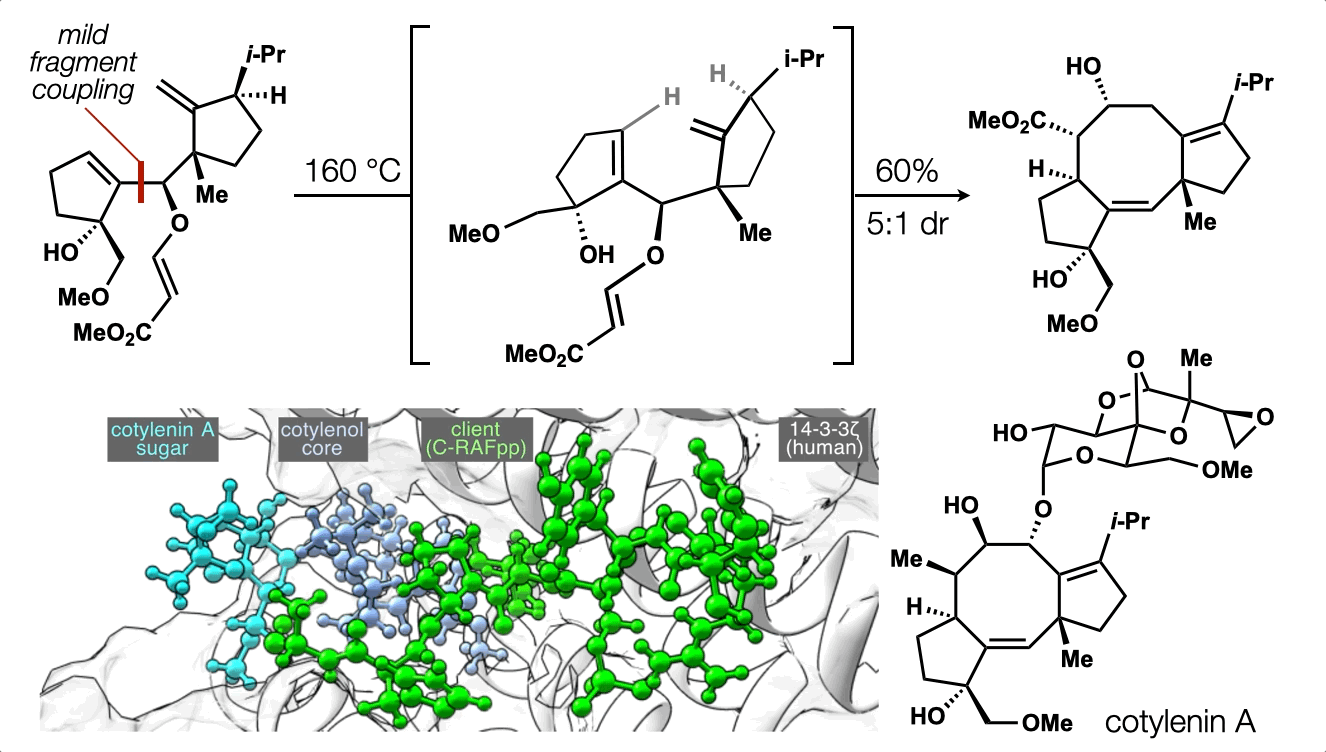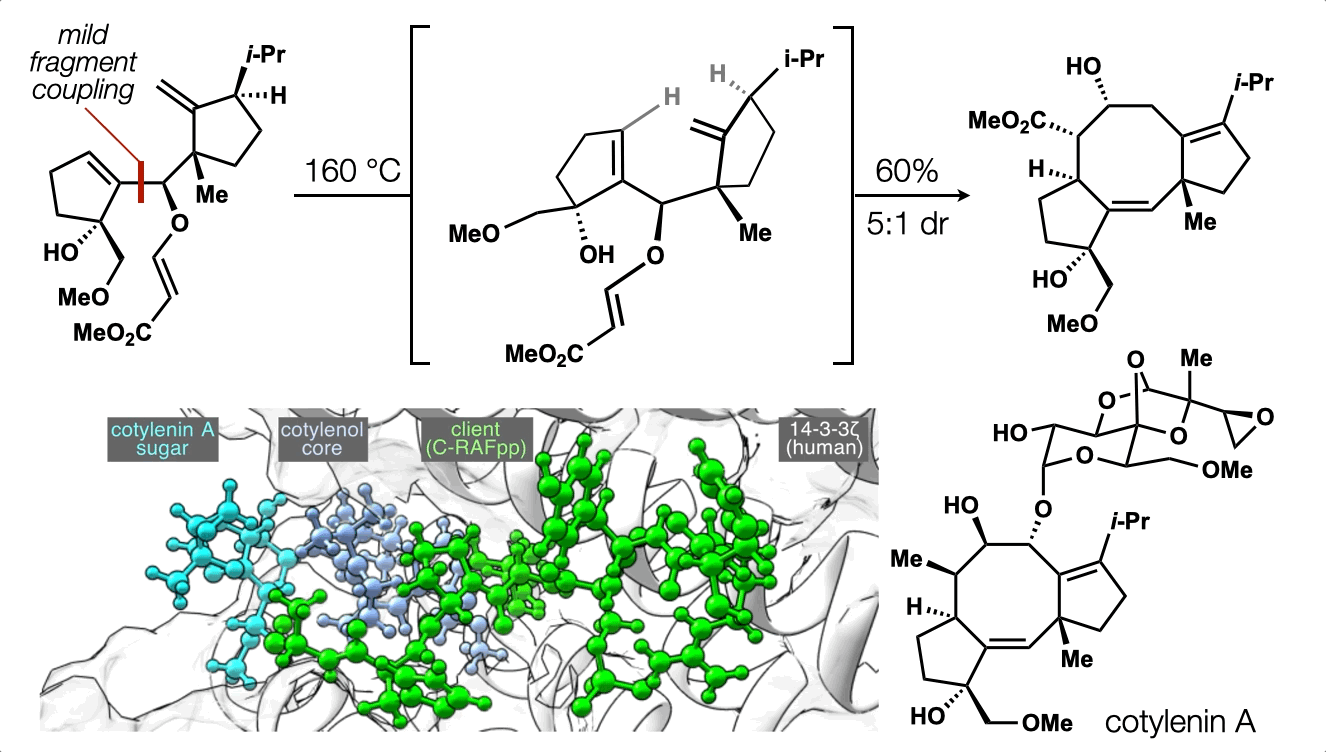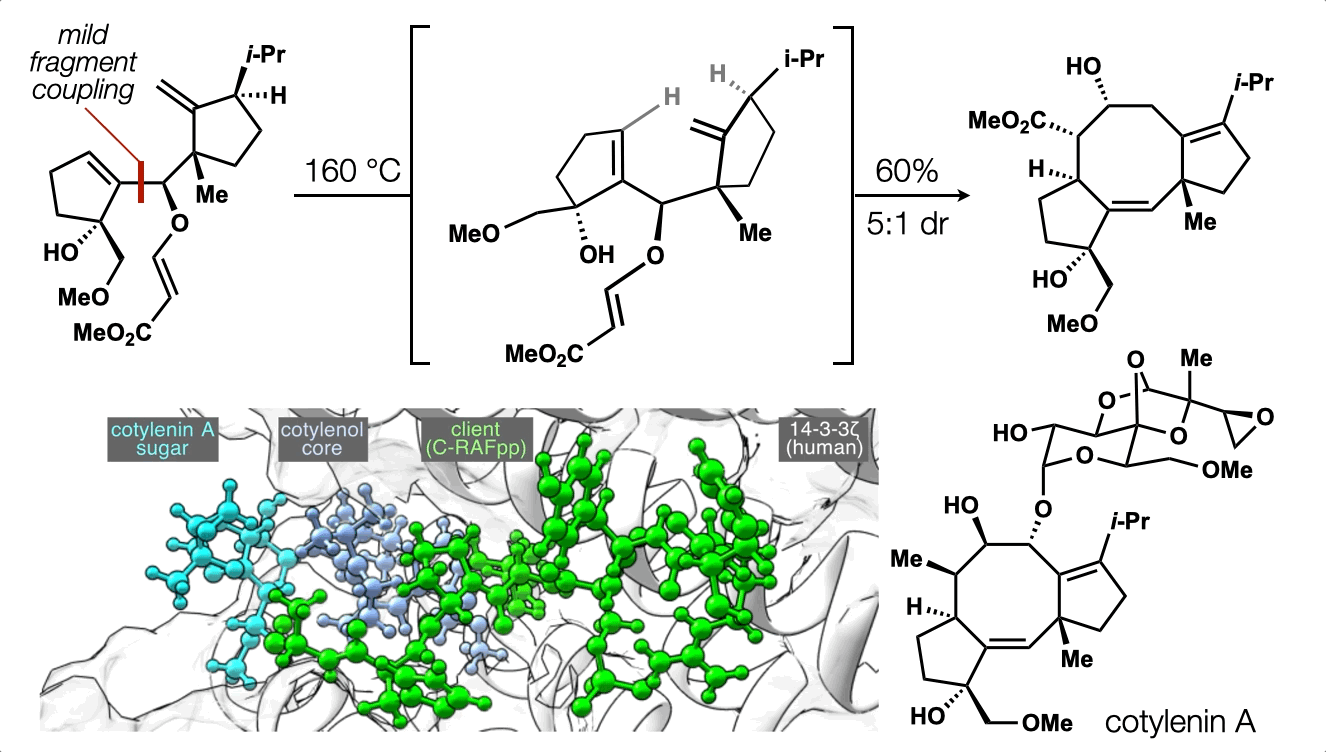
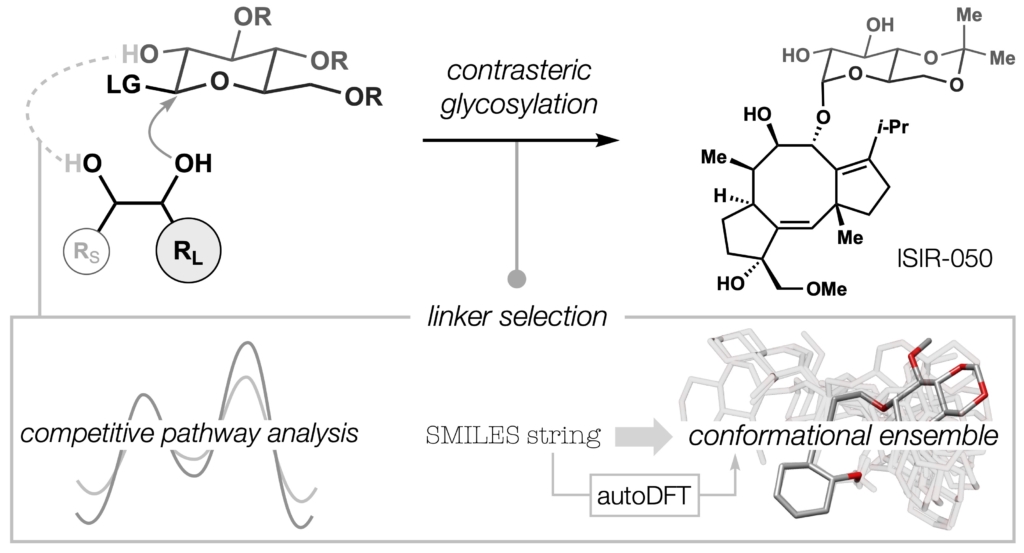
86. Snelson, D.;† Ting, S.;† Shenvi, R. A. “Contrasteric glycosylations of cotylenol and 1,2-diols by virtual linker selection” J. Am. Chem. Soc. 2025, 147, 1327–1333.
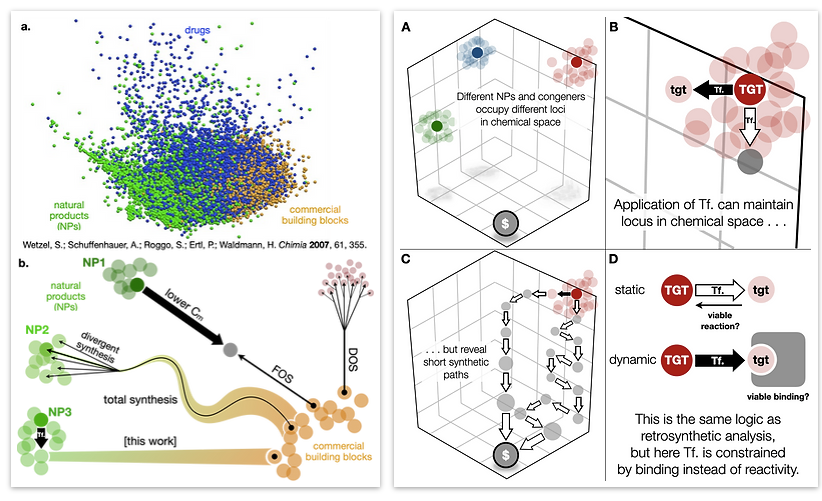
85. Li, C.; Shenvi, R. A. “Total synthesis of twenty-five picrotoxanes by virtual library selection” Nature, 2025, 638, 980–986. ChemRxiv DOI: 10.26434/chemrxiv-2024-g8j1r
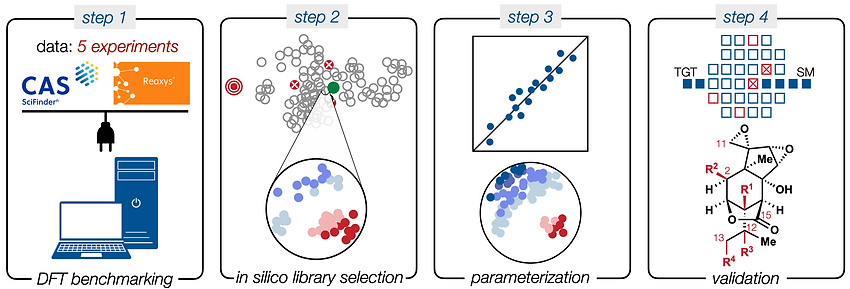
• Nature Magazine Research Briefing
• Document trail to show calculations were predictive, not post hoc
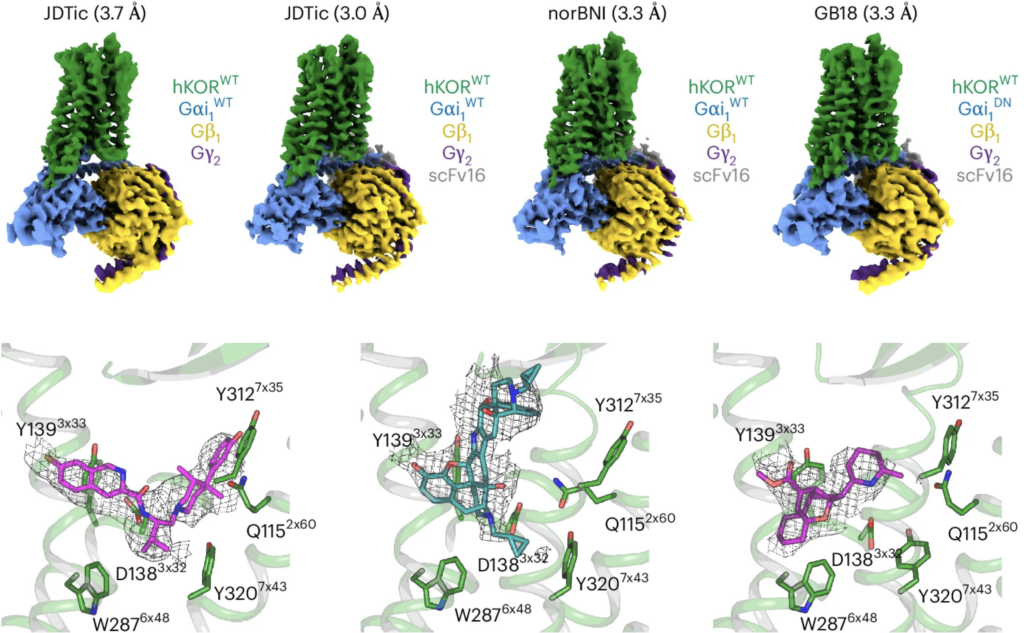
84. Motiwala, Z.; Tyson, A. S.; Styrpejko, D.; Han, G. W.; Khan, S.; Ramos-Gonzalez, N.; Woo, S.; Villers, K.; Landaker, D.; Shenvi, R. A.; Majumdar, S.; Gati, C. “Structural basis of inverse agonism via inactive kappa opioid receptor:G protein complexes” Nature Chem. Bio. 2025, 21, 1046–1057.
2024
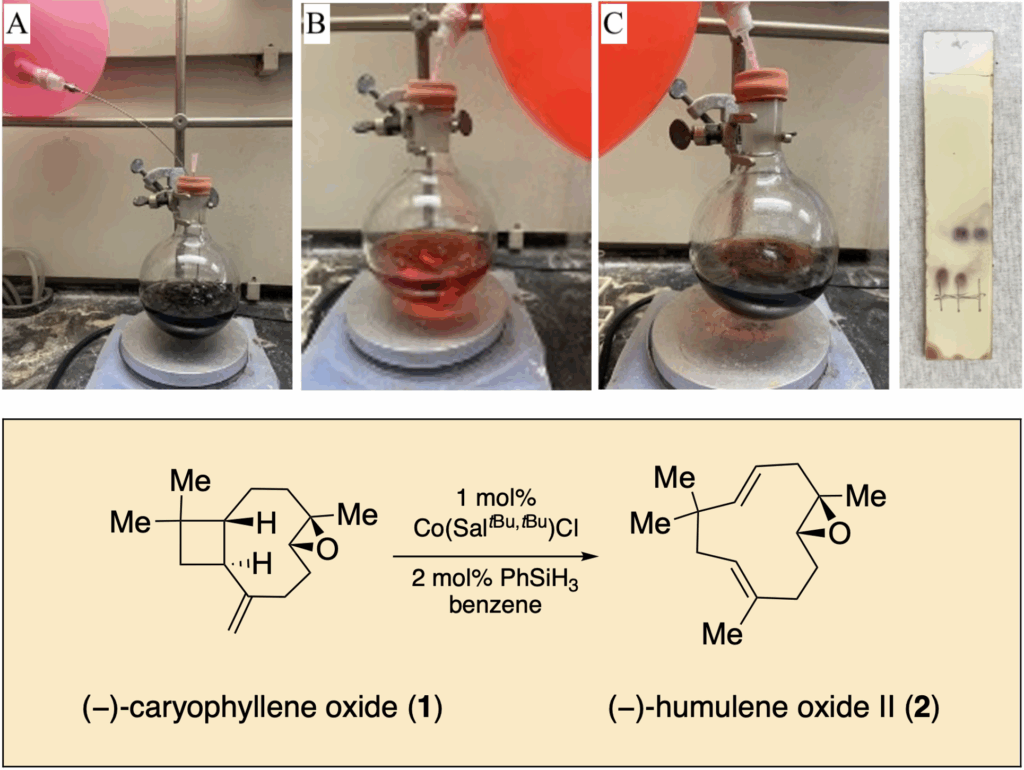
83. Landwehr, E. M.; Shenvi, R. A. “Cobalt-catalyzed radical olefin isomerization: retrocycloisomerization of (–)-caryophyllene oxide to (−)-humulene oxide II” Org. Syn. 2024, 101, 51–60.
[Original protocol in our JACS 2014 16788 paper]
82. Clay, K.; Shenvi, R. A. “The original caretakers of salvinorin A” Nature Chem. 2024, 16, 1735–1736.
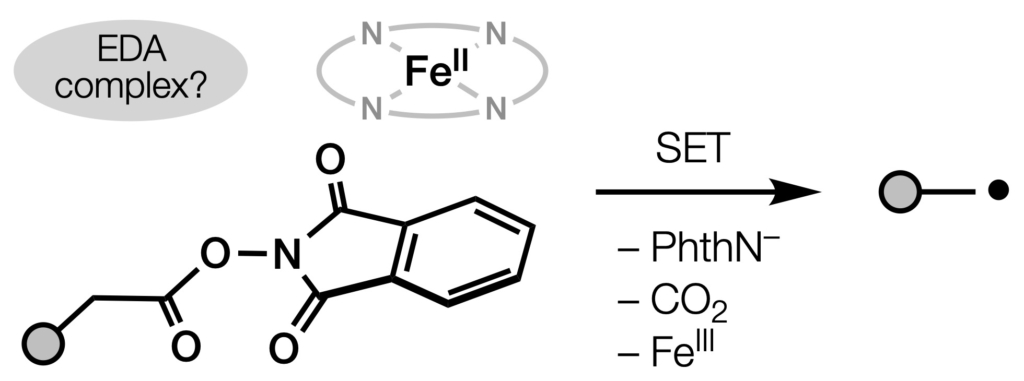
81. Dao, N.; Shenvi, R. A. “On the role of thermally activated EDA complexes in decarboxylative cross-coupling” Tetrahedron [invited, T. Maimone award issue], 2024, 166, 134204.
80. Dao, N.; Gan, X.-c.; Shenvi, R. A. “Metal-Hydride C–C Cross-Coupling of Alkenes Through a Double Outer-Sphere Mechanism” [invited] J. Org. Chem. 2024, 89, 16106–16113.

79. Shenvi, R. A. “Natural product synthesis in the 21st century: beyond the mountain top” [invited Perspective] ACS Cent. Sci. 2024, 10, 519–528.

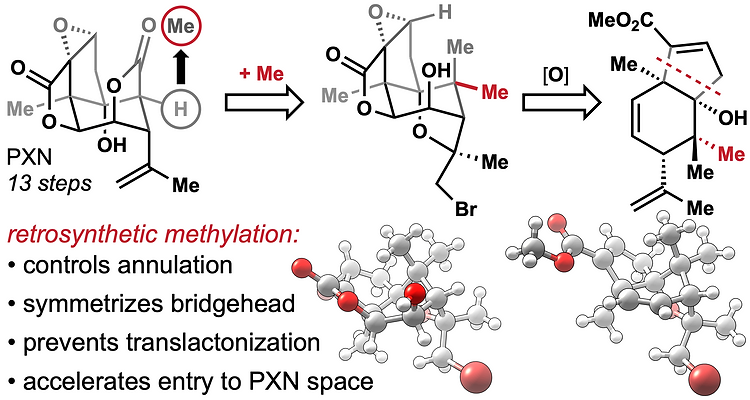
78. Tong, G.; Griffin, S.; Sader, A.; Crowell, A. B.; Beavers, K.; Watson, J.; Buchan, Z.; Chen, S.; Shenvi, R. A. C5 Methylation Confers Accessibility, Stability and Selectivity to Picrotoxinin Nature Commun. 2023, 14, 8308.
ChemRxiv DOI: 10.26434/chemrxiv-2022-0l4nt
77. Gan, X.-c.;† Zhang, B.;† Dao, N.;† Bi, C.; Pokle, M.; Kan, L.; Collins, M. R.; Tyrol, C. C.; Bolduc, P. N.; Nicastri, M.; Kawamata, Y.; Baran, P. S.; Shenvi, R. A. Carbon Quaternization of Redox Active Esters and Olefins via Decarboxylative Coupling. Science 2024, 384, 113. †co-first authors
76. Kong, L.;† Gan, X.-c.;† van der Puyl-Lovett, V. A.;† Shenvi, R. A. Alkene hydrobenzylation by a single catalyst that mediates iterative outer-sphere steps. J. Am. Chem. Soc. 2024, 146, 2351.
ChemRxiv 10.26434/chemrxiv-2023-zw9c2 2023, †co-first authors.
2023
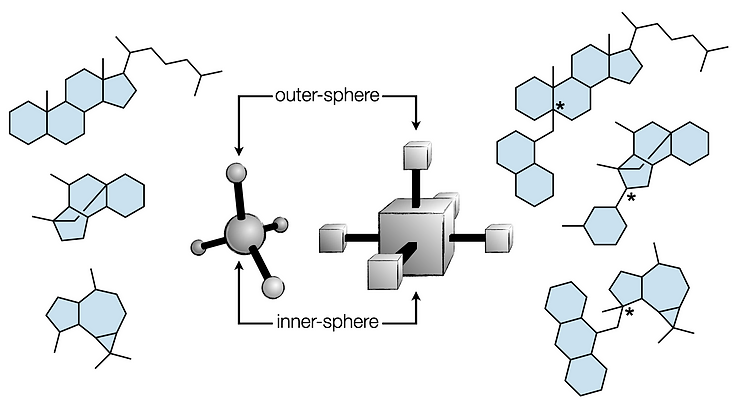
75. Kotesova, S.; Shenvi, R. A. Inner- and outer-sphere cross-couplings for high Fsp3 fragments in natural product space. Acc. Chem. Res. 2023, 56, 3089.
74. Ting, S.;† Snelson, D.;† Huffman, T. R.; Kuroo, A.; Sato, R.; Shenvi, R. A. Synthesis of (–)-cotylenol, a 14-3-3 molecular glue component. J. Am. Chem. Soc. 2023, 145, 20634. †co-first authors
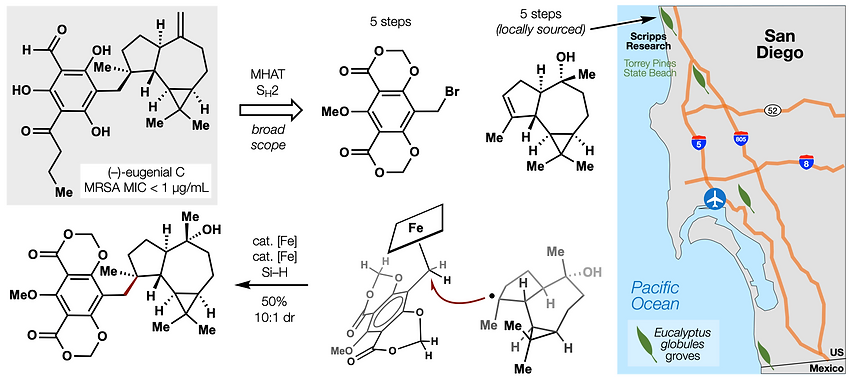
73. Gan, X.-c.;§ Kotesova, S.;§ Castanedo, A.; Green, S. A.; Møller, S. L. B.; Shenvi, R. A. Iron-catalyzed hydrobenzylation: stereoselective synthesis of (–)-eugenial C. J. Am. Chem. Soc. 2023, 145, 15714. §co-first authors
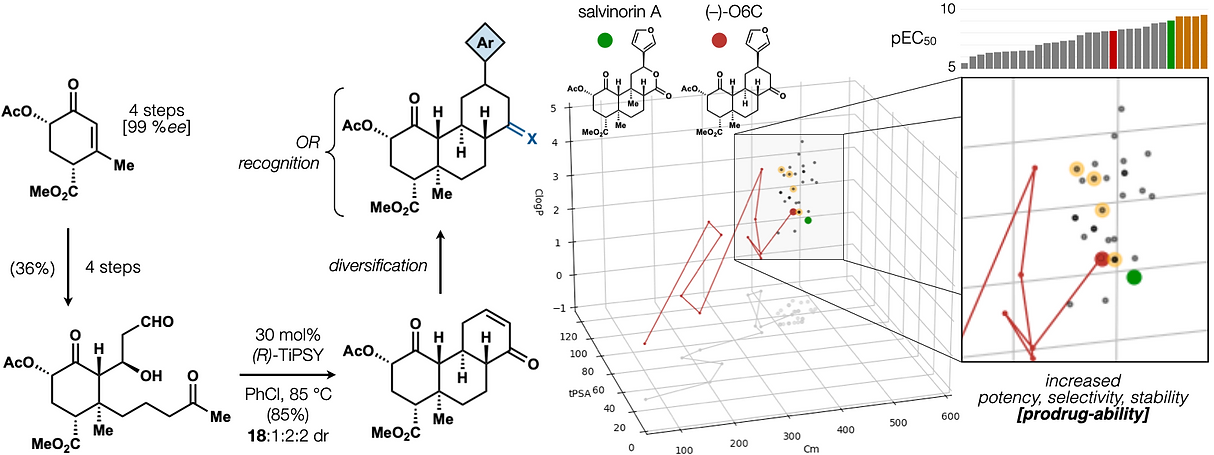
72. Hill, S. J.§; Dao, N.§; Dang, V.; Stahl, E.; Bohn, L. Shenvi, R. A. A route to potent, selective and biased salvinorin chemical space. ACS Cent. Sci., 2023, online. §co-first authors
71. Shenvi, R. A. Early Childhood Care as an Academic: The Slow Burn. Angew. Chem. Int. Ed., 2023, in press [Ihr verborgenes Leben (Their Hidden Lives) Essay Series].

70. van der Puyl, V. A.; Shenvi, R. A. Manganese, iron and cobalt-catalyzed radical olefin hydrofunctionalization Science of Synthesis, 2.14: Base Metal Catalysis, 2023, Ed. N. Yoshikai.
2022
69. Woo, S.; Landwehr, E.; Shenvi, R. A. Synthesis of psychotropic alkaloids from Galbulimima Tetrahedron 2022, 126, 133064. [In celebration of the 65th anniversary of Tetrahedron Publications]
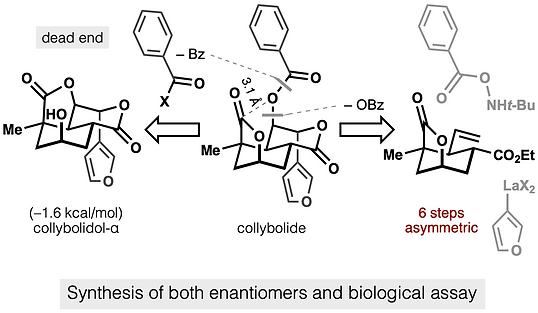
68. Shevick, S. L.; Freeman, S.; Tong, G.; Russo, R. J.; Bohn, L. M.; Shenvi, R. A. Asymmetric syntheses of (+)- and (–)-collybolide enable re-evaluation of kappa-opioid receptor agonism ACS Cent. Sci., 2022, 8, 948–954. DOI: https://doi.org/10.1021/acscentsci.2c00442.
ChemRxiv DOI: 10.26434/chemrxiv-2021-rtxqf
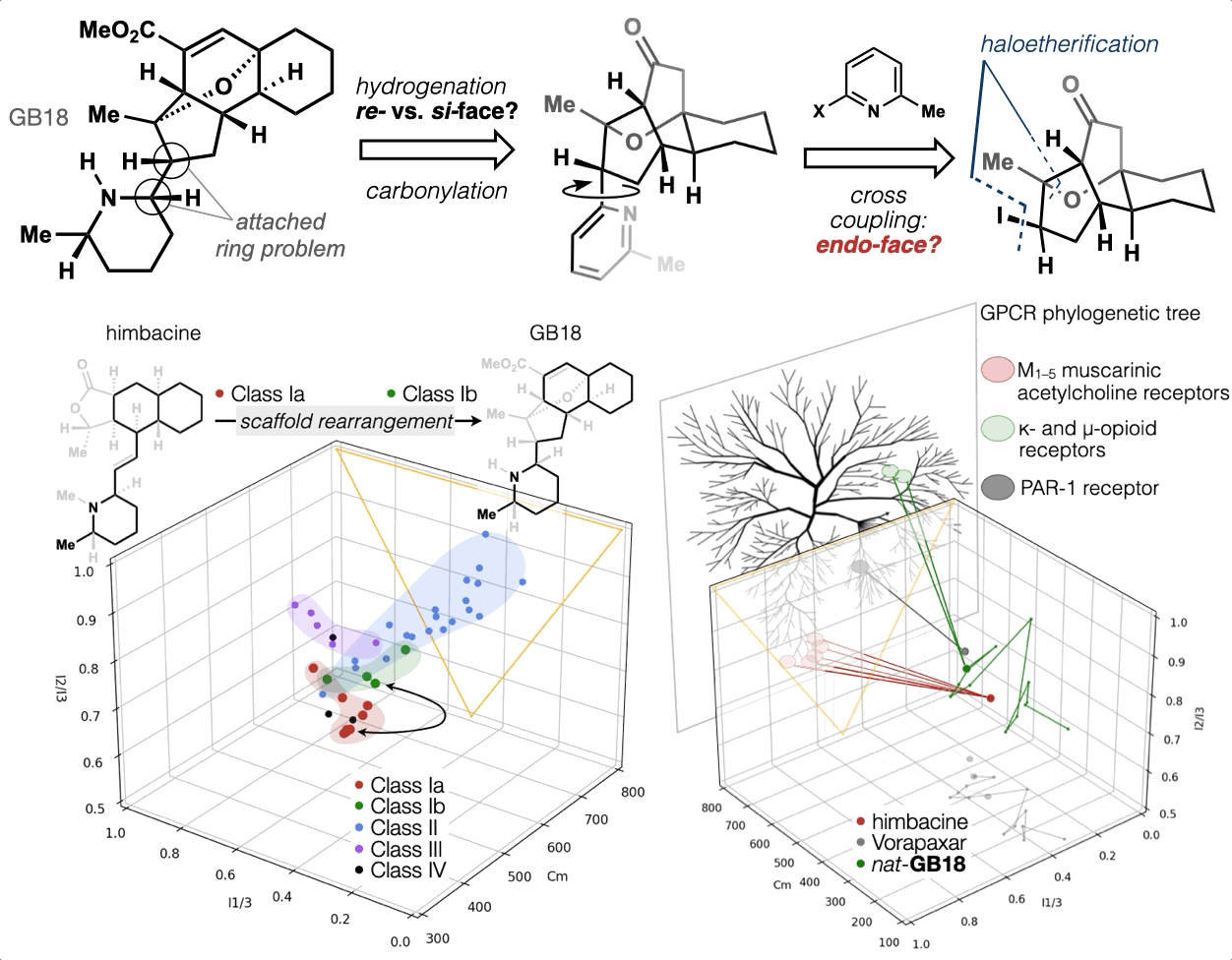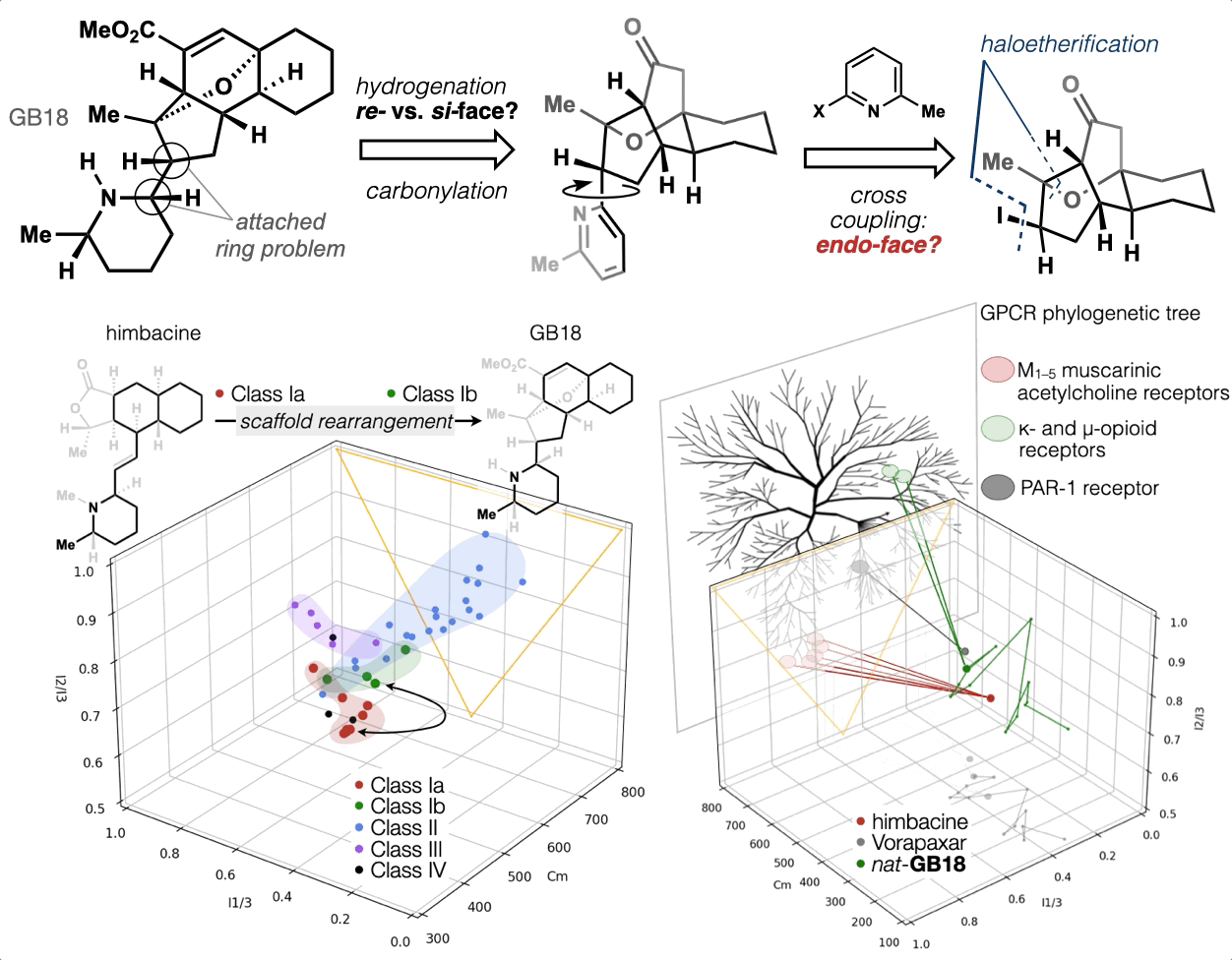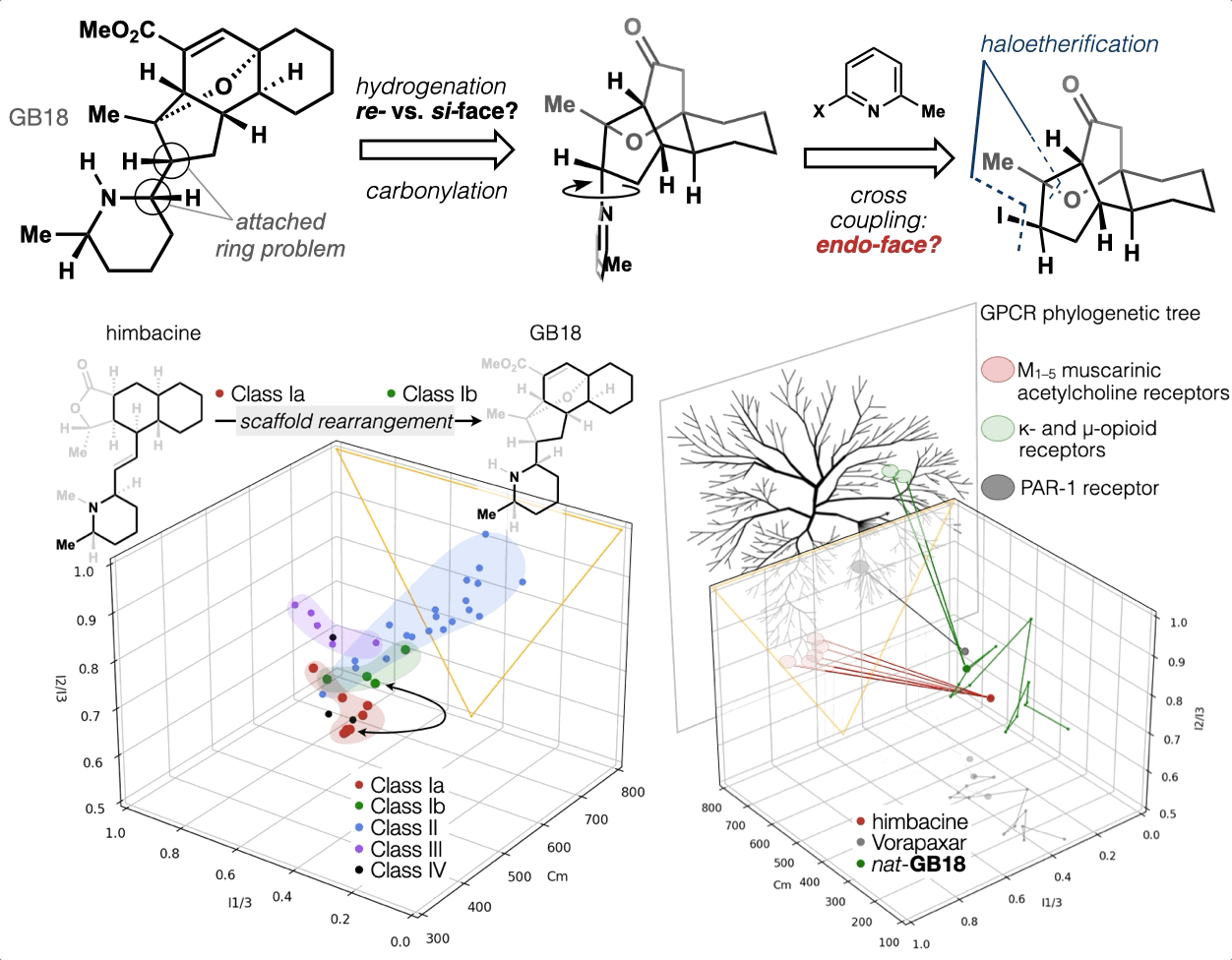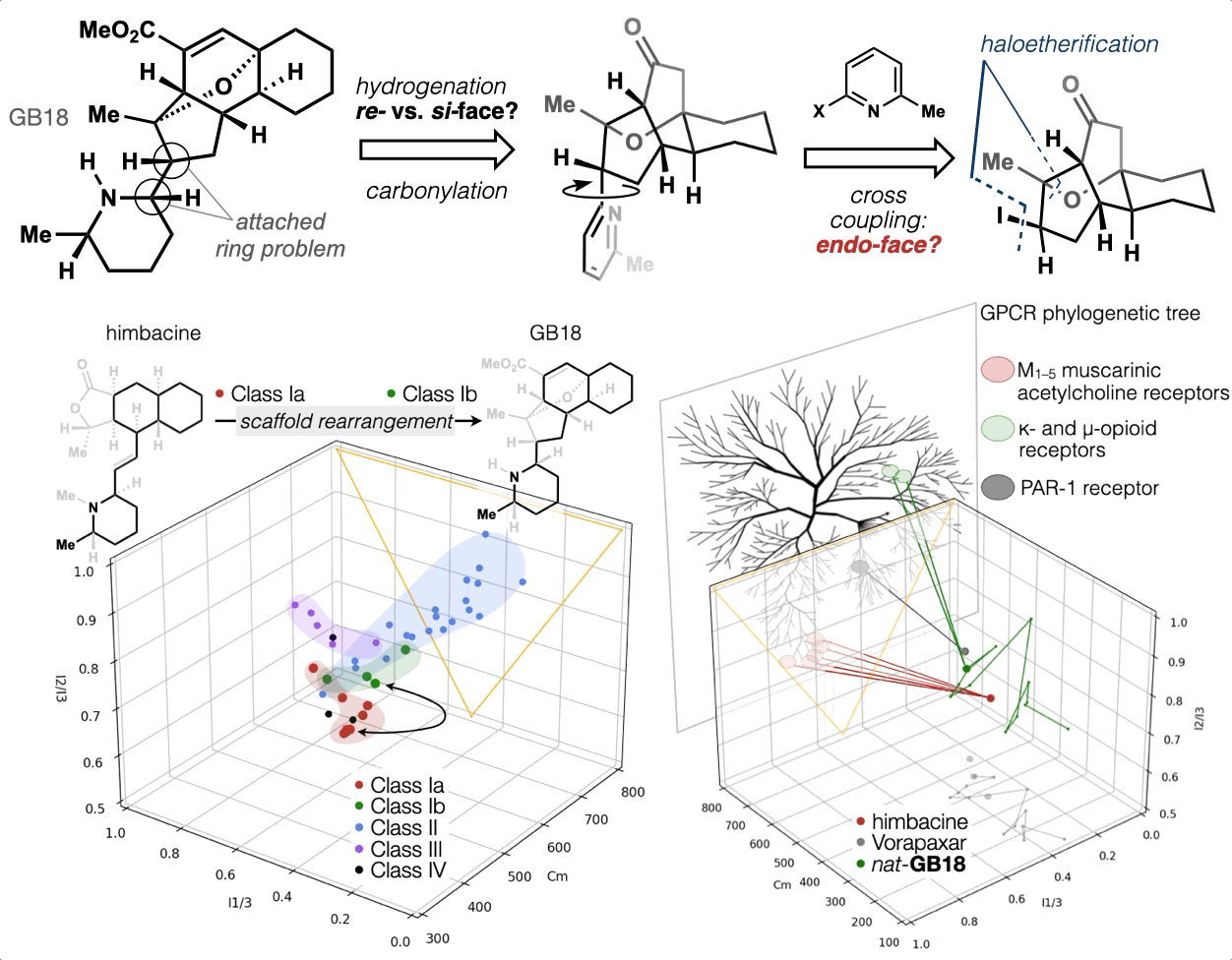
67. Woo, S.; Shenvi, R. A. Synthesis and target annotation of GB18. Nature, 2022, DOI: 10.1038/s41586-022-04840-9.
ChemRxiv DOI: 10.26434/chemrxiv-2022-cs8v5
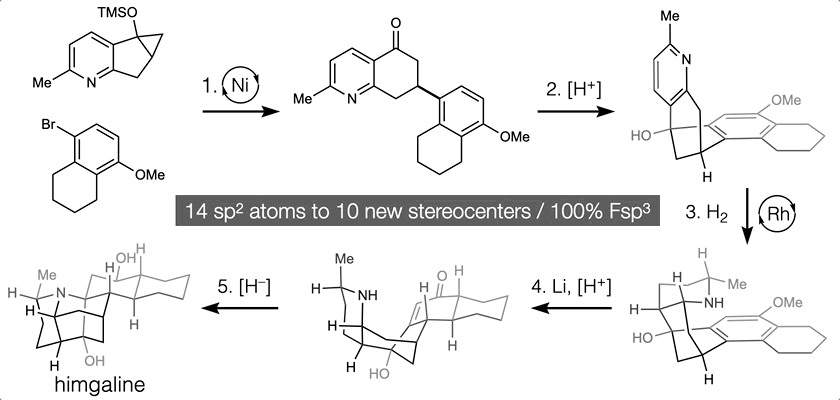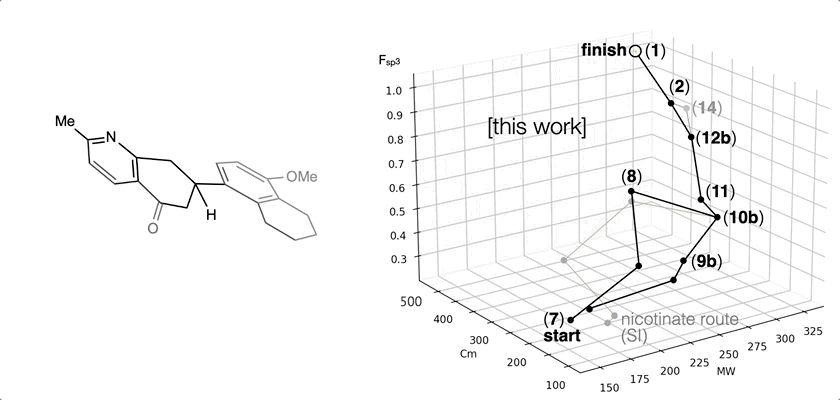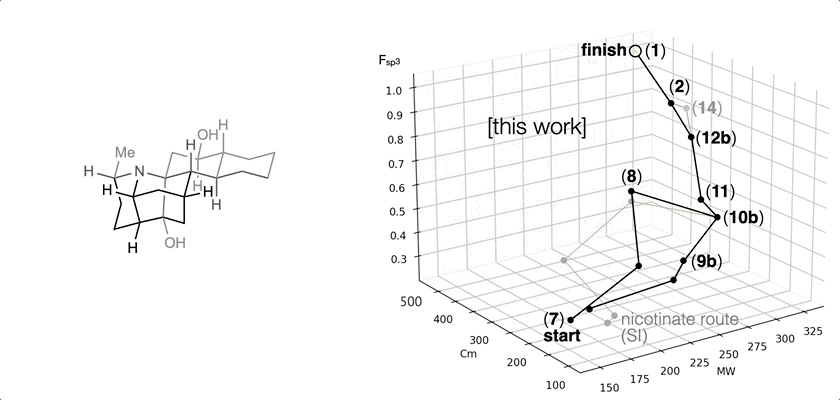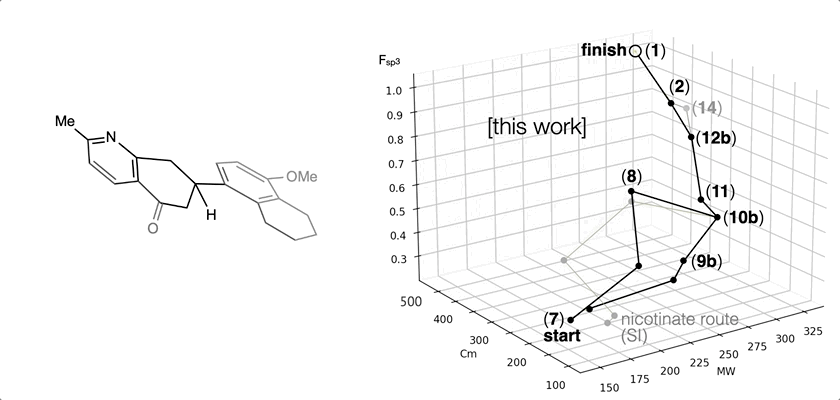
66. Landwehr, E. M.; Baker, M. A.; Oguma, T.; Burdge, H. E.; Kawajiri, T.; Shenvi, R. A. Concise syntheses of GB22, GB13 and himgaline by cross-coupling and complete reduction. Science, 2022, 375, 1270–1274.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
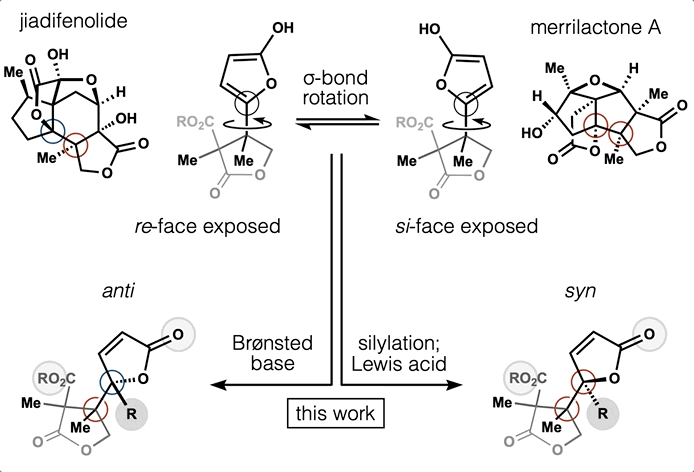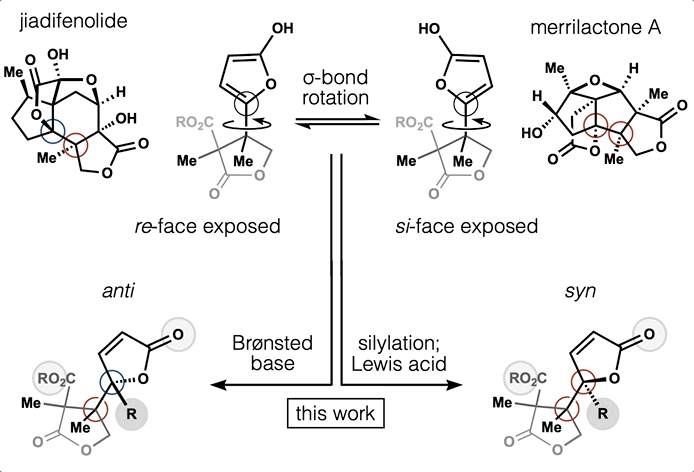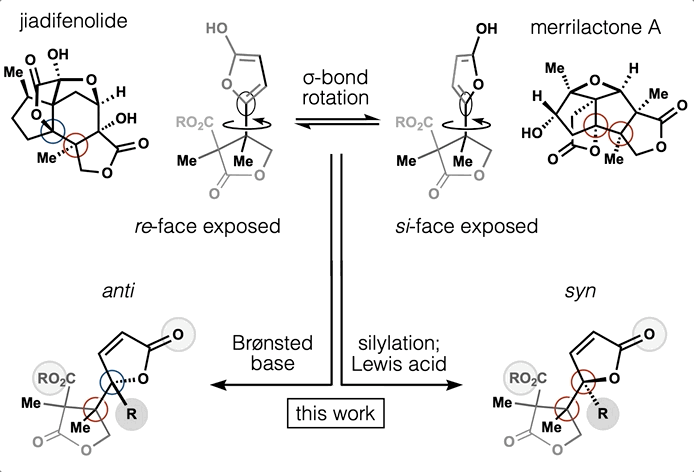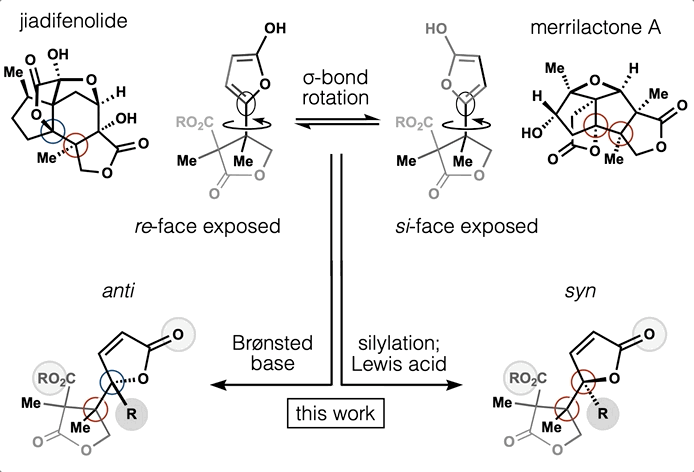
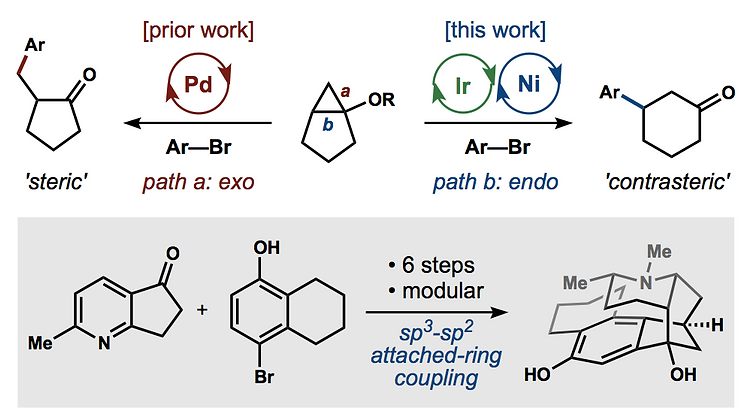
65. Huffman, B. J.; Chu, T.; Hanaki, Y.; Wong, J. J.; Chen, S.; Houk, K. N.; Shenvi, R. A. Stereodivergent attached ring synthesis via non-covalent interactions: a short formal synthesis of merrilactone A Angew. Chem. Int. Ed. 2022, 61, e202114514.
2021
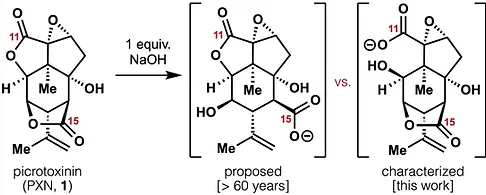
64. Tong, G.; Shenvi, R. A. Revision of the unstable picrotoxinin hydrolysis product Angew. Chem. Int. Ed. 2021, 60, 19113.
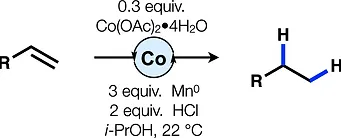
63. van der Puyl, V.; McCourt, R. O.; Shenvi, R. A. Cobalt-catalyzed alkene hydrogenation by reductive turnover Tetrahedron Lett. 2021, 72, 153047. On the occasion of the 2020 Tetrahedron Prize to Dale Boger.
62. Woo, S.; Shenvi, R. A. Natural Product Synthesis through the Lens of Informatics Acc. Chem. Res. 2021, 54, 1157–1167.
2020
61. Tong, G.; Baker, M. A.; Shenvi, R. A. Change the channel: CysLoop receptor antagonists from Nature. Pest Manag. Sci. 2020, 77, 3650–3662. [Special Issue honoring Tom Sparks for the Kenneth Spencer Award]
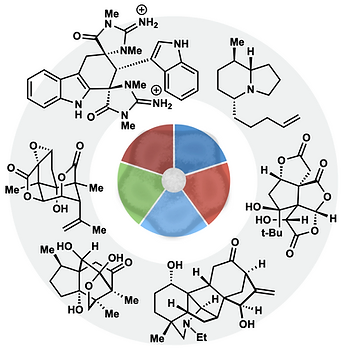
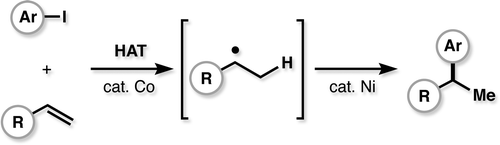
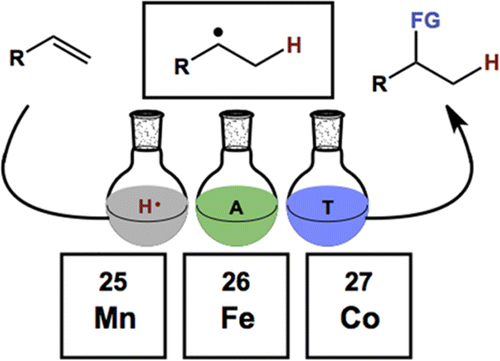
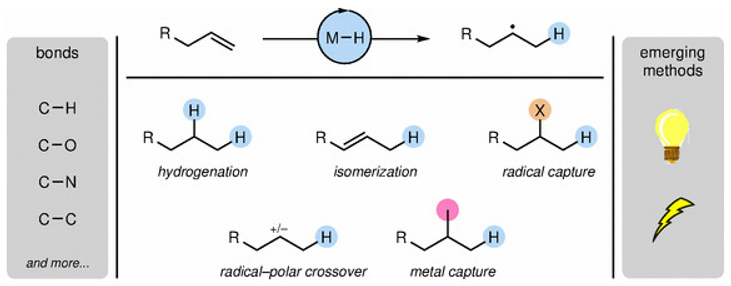
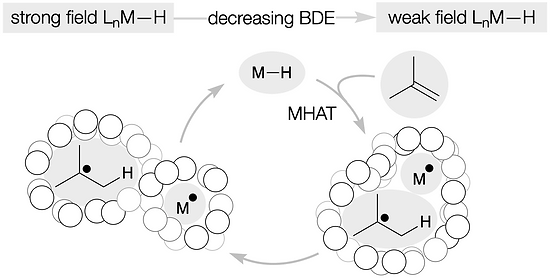
60. Shevick, S. L.; Wilson, C. V.; Kotesova, S.; Kim, D.; Holland, P. L.; Shenvi, R. A. Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals. Chem. Sci. 2020, 12401–12422.
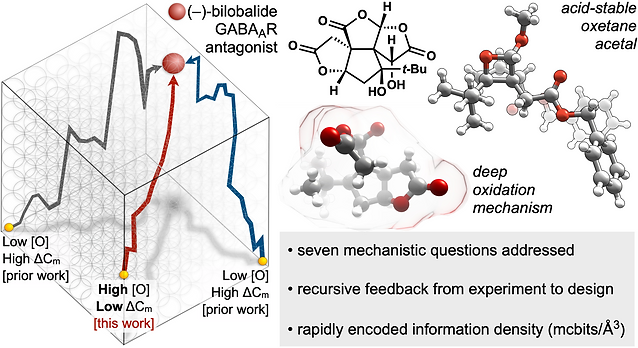
59. Demoret, R. M.; Baker, M. A.; Ohtawa, M.; Chen, S.; Lam, C.-C.; Khom, S.; Roberto, M.; Forli, S.; Houk, K.; Shenvi, R. A. Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide. J. Am. Chem. Soc. 2020, 142, 18599–18618.ChemRxiv DOI: 10.26434/ chemrxiv.12132939.v2
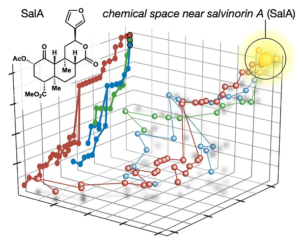
58. Hill, S. J.; Brion, A. U. C. M.; Shenvi, R. A. Chemical Syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020, 37, 1478–1496.

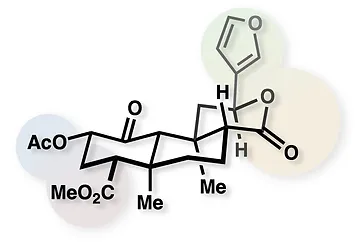
57. Crossley, S. W. M.; Tong, G.; Lambrecht, M. J.; Burdge, H. E.; Shenvi, R. A. Total Synthesis of (–)-Picrotoxinin J. Am. Chem. Soc. 2020, 142, 11376–11381.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
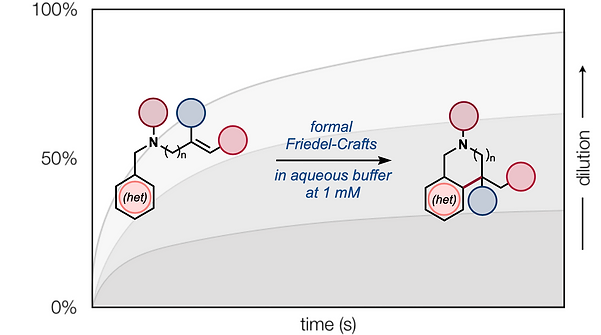
56. Matos, J. L. M.; Green, S. A.; Chun, Y; Dang, V. Q.; Dushin, R. G.; Richardson, P.; Chen, J. S.; Piotrowski, D. W.; Paegel, B. M.; Shenvi, R. A. Cycloisomerization of olefins in water, Angew. Chem. Int. Ed. 2020, 59, 12998–13003.
ChemRxiv: 10.26434/chemrxiv.11977917.v1
55. Burdge, H. E.; Oguma, T.; Kawajiri, T.; Shenvi, R. A. Concise Synthesis of GB22 by Endo-Selective Siloxycyclopropane Arylation ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1 .
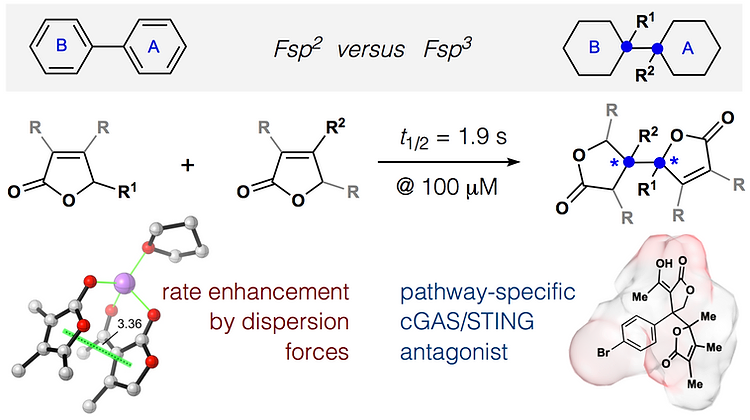
54. Huffman, B. J.; Chen, S.; Schwarz, J. L; Plata, R. E.; Chin, E.; Houk, K. N.; Lairson, L. L.; Shenvi, R. A. Electronic Complementarity Permits Hindered Butenolide Heterodimerization and Discovery of Novel cGAS/STING Pathway Antagonists, Nature Chem. 2020, 12, 310–317.
2019
53. Shevick, S. L.; Baker, M. A.; Shenvi, R. A. Alkene Hydroarylation by Co/Ni Dual Catalysis, Cell: Trends in Chemistry, 2019, 1, 540–541.
52. Matos, J. L. M.; Green, S. A.; Shenvi, R. A. Chapter 7. Markovnikov Functionalization by Hydrogen Atom Transfer, Organic Reactions, 2019, 100, 383–470.
51. Baker, M.; Demoret, R.; Ohtawa, M.; Shenvi, R. A. Concise Asymmetric Synthesis of (–)-Bilobalide Nature 2019, 575, 643–646. DOI: 10.1038/s41586-019-1690-5. ChemRxiv DOI: 10.26434/chemrxiv.8202053.v1 Featured in Org. Chem. Highlights
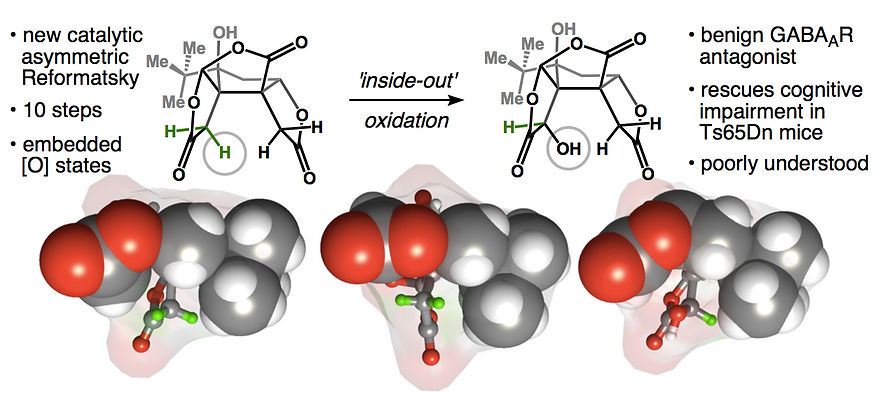
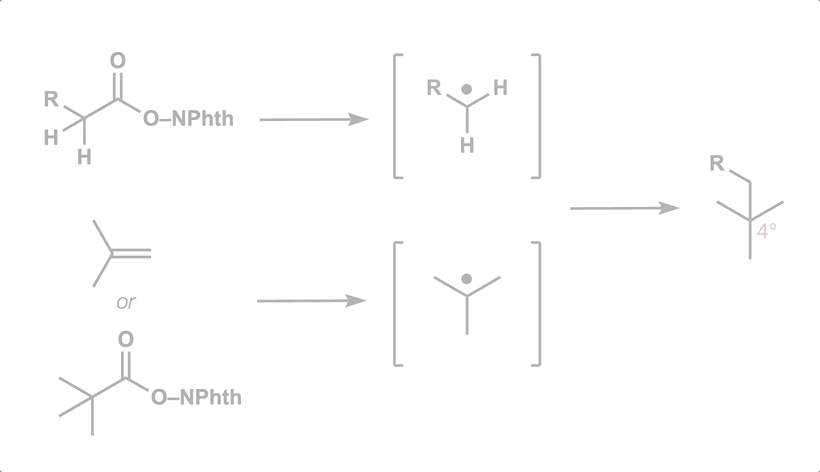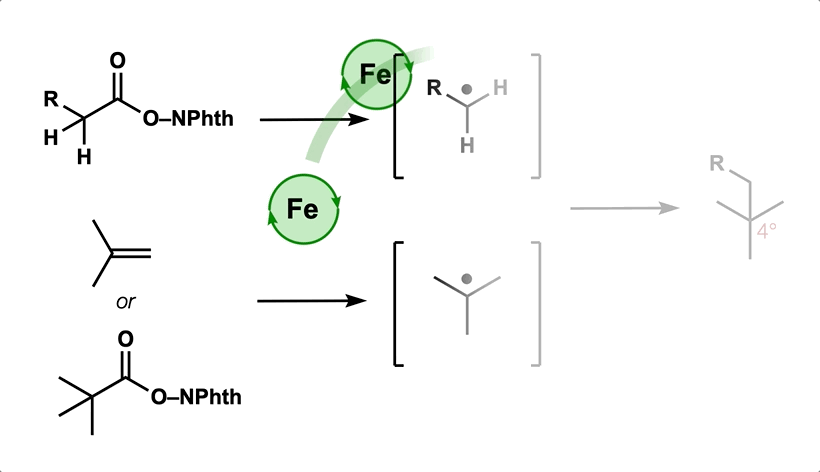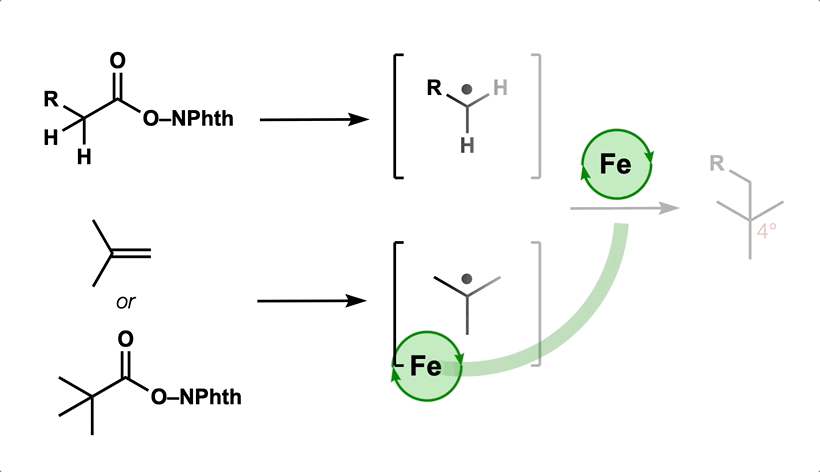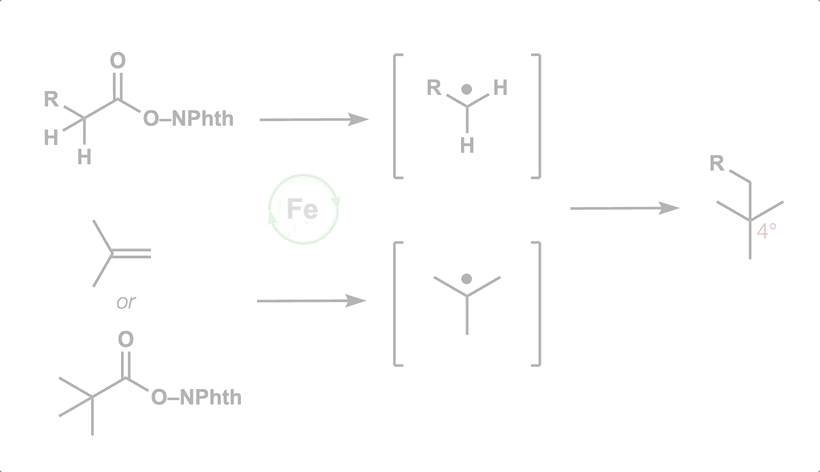
50. Green, S. A.; Huffman, T. R.; McCourt, R. O.; van der Puyl, V.; Shenvi, R. A. Hydroalkylation of Olefins to form Quaternary Carbons J. Am. Chem. Soc. 2019, 141, 7709-7714.
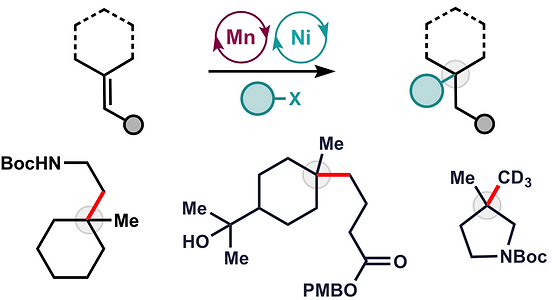
49. Martinez, R.; Burdge, H.; Shenvi, R. A. Reanalysis of Lindenatriene, a Building Block for the Synthesis of Lindenane Oligomers Tetrahedron 2019, 75, 3140-3144. [invited manuscript for Ryan’s Tetrahedron Young Investigator Award 2019]
48. Huffman, B.; Shenvi, R.A. Natural Products in the ‘Marketplace’: Interfacing Synthesis and Biology J. Am. Chem. Soc. 2019, 141, 3332-3346.
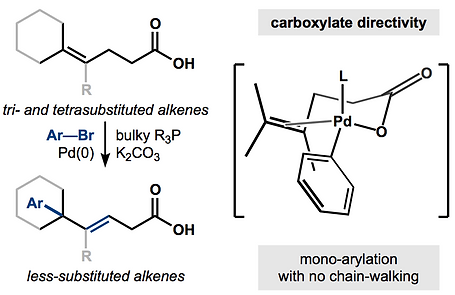
47. Huffman, T.; Wu, Y.; Emmerich, A.; Shenvi, R.A. Intermolecular Heck Coupling with Hindered Alkenes Directed by Potassium Carboxylates Angew. Chem. Int. Ed. 2019, 58, 2371-2376.
2018
46. Matos, J.L.M.; Vásquez-Céspedes, S.; Gu, J.; Oguma, T.; Shenvi, R.A. Branch-Selective Addition of Unactivated Olefins into Imines and Aldehydes J. Am. Chem. Soc. 2018, 140, 16976–16981.
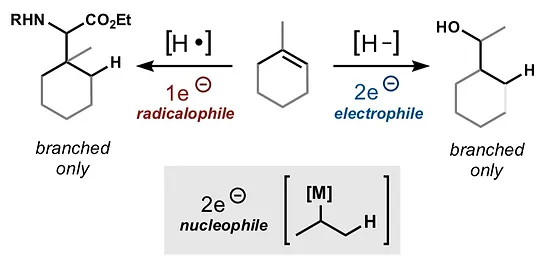
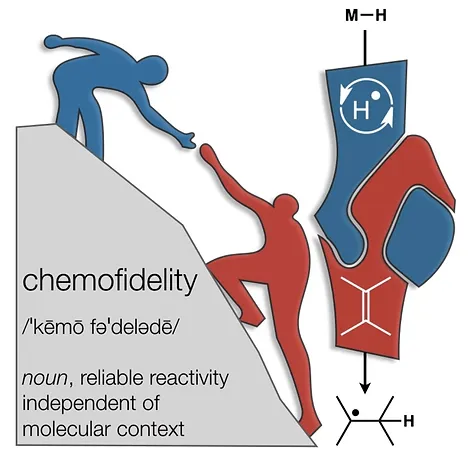
45. Green, S.A.; Crossley, S.W.M.; Matos, J.L.M.; Vásquez-Céspedes, S.; Shevick, S. L.; Shenvi, R. A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer Acc. Chem. Res., 2018, 51, 2628–2640.
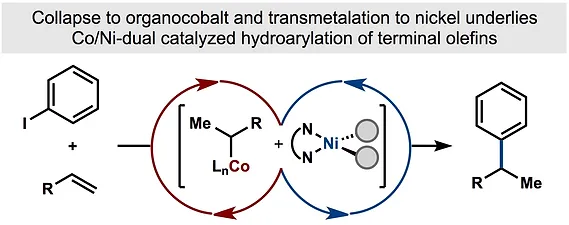
44. Shevick, S. L.; Obradors, C.; Shenvi, R. A. Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation J. Am. Chem. Soc. 2018,140, 12056–12068.
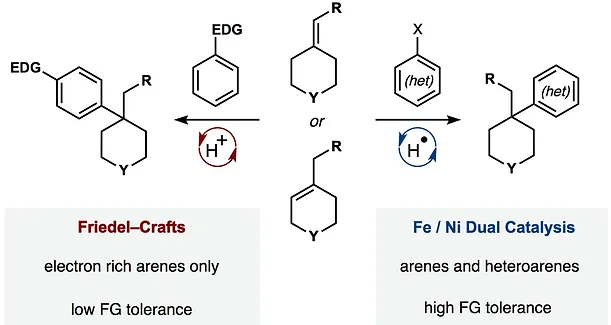
43. Green, S. A.; Vásquez-Céspedes, S.; Shenvi, R. A. Iron-Nickel Dual-Catalysis: A New Engine for Olefin Functionalization. J. Am. Chem. Soc. 2018, 140, 11317–11324
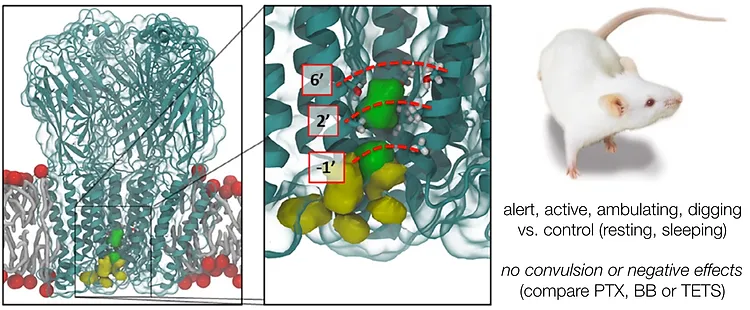
42. Jeffrey M. Witkin, Ryan A. Shenvi, Xia Li, Scott D. Gleason, Julie Weiss, Denise Morrow, John T. Catow, Mark Wakulchik, Masaki Ohtawa, Hai-Hua Lu, Michael D. Martinez, Jeffrey M. Schkeryantz, Timothy S. Carpenter, Felice C. Lightstone, Rok Cerne Pharmacological characterization of the neurotrophic sesquiterpene jiadifenolide reveals a non-convulsant signature and potential for progression in neurodegenerative disease studies. Biochem. Pharm. 2018, 155, 61–70.
DOI: 10.1016/j.bcp.2018.06.022
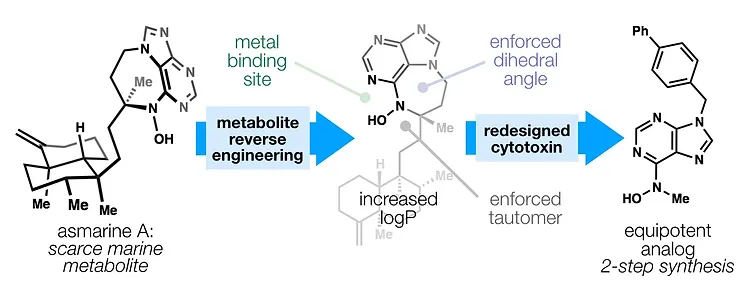
41. Lambrecht, M.; Kelly, J. W.; Shenvi, R. A. Mechanism of Action of the Cytotoxic Asmarine Alkaloids. ACS Chem. Bio. 2018, 13, 1299–1306. DOI: 10.1021/acschembio.8b00096
40. Roach, J. J.; Shenvi, R. A. A Review of Salvinorin Analogs and their Kappa-Opioid Receptor Activity. Bioorg. Med. Chem. Lett. 2018, 28, 1436–1445. DOI: 10.1016/j.bmcl.2018.03.029
2017
39. Hirasawa, S.; Cho, M.; Brust, T. F.; Roach, J. J.; Bohn, L. M.; Shenvi, R. A. O6C-20-nor-SalA is a stable and potent KOR agonist. Bioorg. Med. Chem. Lett. 2018, 28, 2770–2722. Special Issue dedicated to Dale Boger.
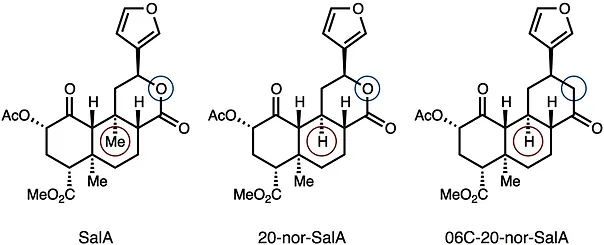
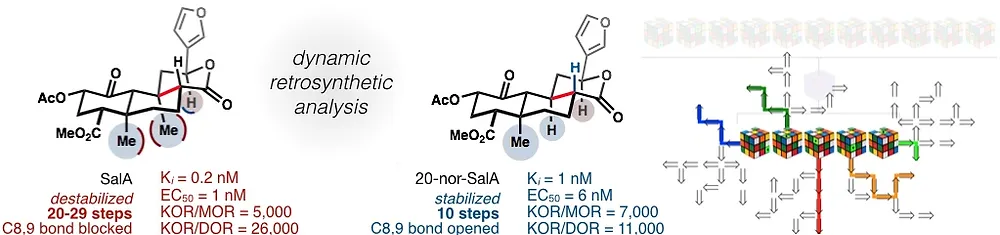
38. Roach, J. J.; Sasano, Y.; Schmid, C. L.; Zaidi, S.; Katrich, V.; Stevens, R. C.; Bohn, L. M.; Shenvi, R. A. Dynamic Strategic Bond Analysis Yields a 10-step Synthesis of 20-nor-SalA, a Potent Κ-OR Agonist. A(CS)2 2017, 3, 1329–1336.
ChemRxiv DOI: 10.26434/chemrxiv.5318188
- #1 Most Viewed Article on ChemRxiv, Aug-Dec 2017.
- Highlighted by Tien Ngyuen in C&EN, Sept. 1 2017.
- See article on SlashGear by Brittany Rosen, Sept. 11, 2017
- See article on Gizmodo by Ryan Mandalbaum, Sept. 12, 2017
- See article on Geek by Daniel Starkey, Sept. 12, 2017
- See article on Wired by Matthew Simon, Jan. 8, 2018
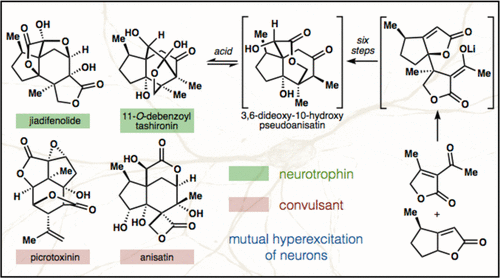
37. Ohtawa, M.; Krambis, M. J.; Cerne, R.; Schkeryantz, J.; Witkin, J. M.; Shenvi, R. A. Synthesis of (–)-11-O-Debenzoyltashironin: Neurotrophic Sesquiterpenes Cause Hyperexcitation. J. Am. Chem. Soc. 2017, 139, 9637-9644.
Featured in Org. Chem. Highlights
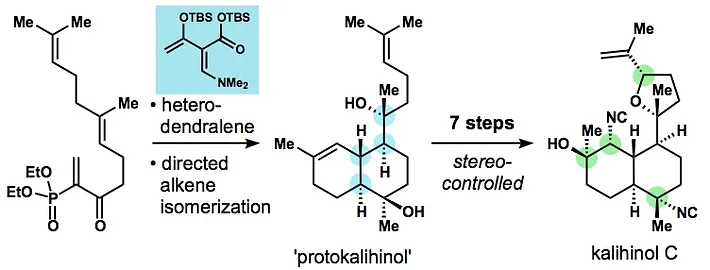
36. Reiher, C. A.; Shenvi, R. A. Stereocontrolled Synthesis of Kalihinol C. J. Am. Chem. Soc. 2017, 139, 3647-3650.
2016
35. Green, S. A.; Matos, J. L. M.; Yagi, A.; Shenvi, R. A. Branch-Selective Hydroarylation: Iodoarene-Olefin Cross Coupling. J. Am. Chem. Soc. 2016, 138, 12779-12782.
34. Shenvi, R. A. And You, of Tender Years. Chem. 2016, 1, 331.
33. Crossley, S. W. M.; Martinez, R. M.; Obradors, C.; Shenvi, R. A. Mn, Fe, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912-9000.
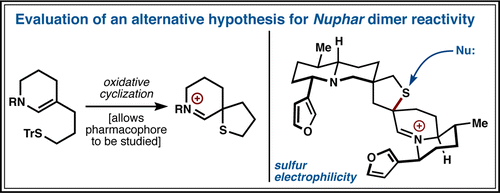
32. Tada, N.; Jansen, D. J.; Mower, M. P.; Blewett, M. M.; Umotoy, J. C.; Cravatt, B. F.; Wolan, D. W.; Shenvi, R. A. Synthesis and Sulfur Electrophilicity of the Nuphar Thiaspirane Pharmacophore. ACS Cent. Sci. 2016, 2, 401-408.
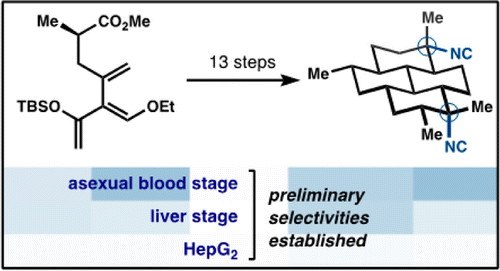
31. Lu, H.-H.; Pronin, S. V.; Antonova-Koch, Y.; Meister, S.; Winzeler, E. A.; Shenvi, R. A. Synthesis of (+)-7,20-Diisocyanoadociane and Liver-Stage Antiplasmodial Activity of the Isocyanoterpene Class. J. Am. Chem. Soc. 2016, 138, 7268-7271.
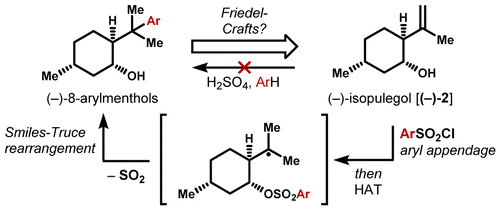
30. Crossley, S. W. M.; Martinez, R. M.; Zuluaga, S. G.; Shenvi R. A. Synthesis of the Privileged 8-Arylmenthol Class by Radical Arylation of Isopulegol. Org. Lett. 2016, 18, 2620-2623.
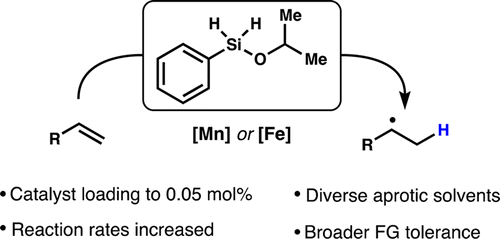
29. Obradors, C. L.; Martinez, R.; Shenvi, R. A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016, 138, 4962-4971.
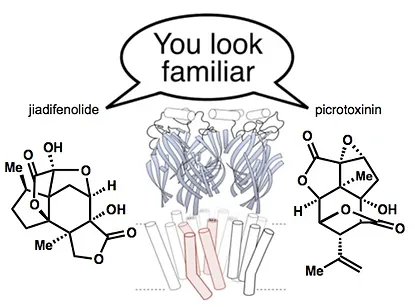
28. Shenvi, R. A. Neurite Outgrowth Enhancement by Jiadifenolide: Possible Targets. Nat. Prod. Rep. 2016, 33, 535-539.
27. Shenvi, R. A. Reinventing Radical Reactions. SynLett Cluster (Ed. T. Rovis and R. A. Shenvi). 2016, 27, 678-679.
26. Wan, K. K.; Shenvi, R. A. Conjuring a Supernatural Product – Delmarine. SynLett (invited Accounts). 2016, 27, 1145-1164.
2015
25. Tabor, M. G.; Shenvi, R. A. Synthesis of Lepadiformine Using a Hydroamination Transform. Org. Lett. 2015, 17, 5776.
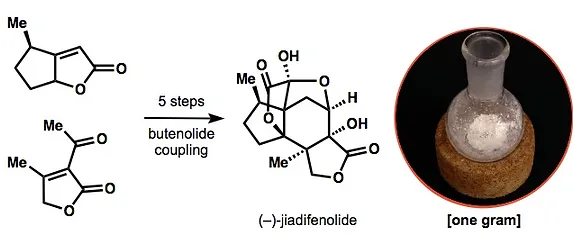
24. Lu, H.-H.; Martinez, M. D.; Shenvi, R. A. Eight-Step, Gram-Scale Synthesis of (–)-Jiadifenolide. Nature Chem. 2015, 7, 604-607.
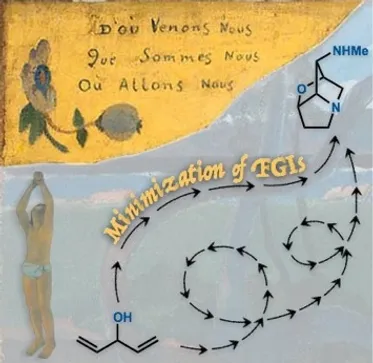
23. Crossley, S. W. M.; Shenvi, R. A. A Longitudinal Study of Alkaloid Synthesis Reveals Functional Group Interconversions (FGIs) as Bad Actors. Chem. Rev. 2015, 115, 9465-9531.
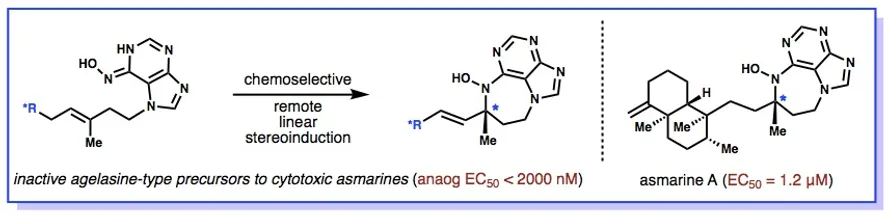
22. Wan, K. K.; Iwasaki, K.; Umotoy, J. C.; Wolan, D.; Shenvi, R. A. Nitrosopurines en route to Potent Asmarine Cytotoxins. Angew. Chem. Int. Ed. 2015, 127, 2440-2445.
21. Shenvi, R. A.; Schnermann, M. J. Syntheses and Biological Studies of Marine Terpenoids Derived from Inorganic Cyanide. Nat. Prod. Rep. 2015, 32, 543-577.
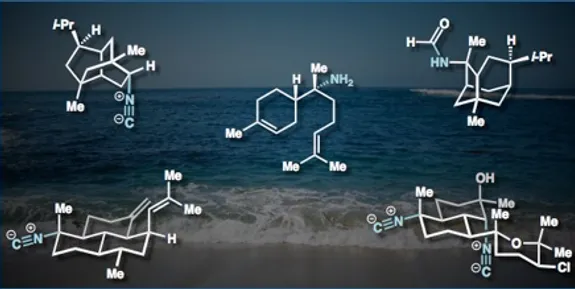
2014
20. Crossley, S. W. M.; Barabé, F.; Shenvi, R. A. Simple, Chemoselective, Catalytic Olefin Isomerization. J. Am. Chem. Soc. 2014, 136, 16788.
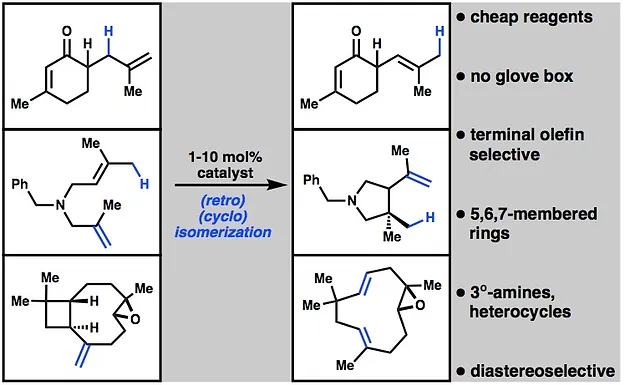
Isomerization pre-catalyst now available from Sigma-Aldrich
19. Jansen, D. J.; Shenvi, R. A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med. Chem., 2014, 6, 1127.
18. Iwasaki, K.; Wan, K. K.; Oppedisano, A.; Crossley, S. W. M.; Shenvi R. A. Simple, Chemoselective Hydrogenation with Thermodynamic Stereocontrol. J. Am. Chem. Soc. 2014, 136, 1300-1303.
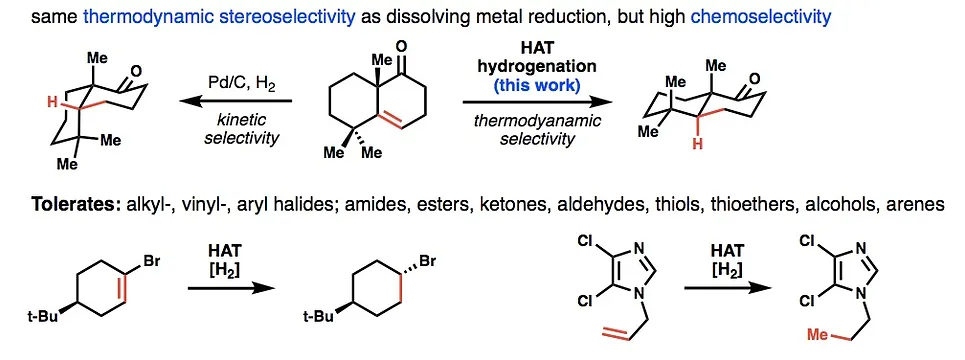
- Hydrogenation catalyst available from Sigma-Aldrich
- Highlighted by Carmen Drahl in C&EN 2014, 92, 9.
2013
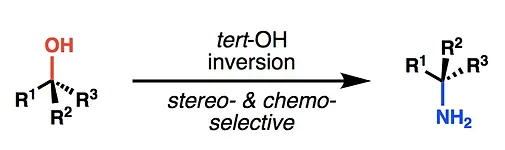
17. Pronin, S. V.; Reiher, C. A.; Shenvi, R. A. Stereoinversion of tertiary alcohols to tertiary alkyl isonitriles and amines. Nature. 2013, 501, 195-199.
- Featured by TCI Chemicals
- Highlighted by Bethany Halford in C&EN 2013, 91, 8.
- Highlighted by Stephen Davey in Nature Chemistry 2013, 5, 808.
- Highlighted by A. Räder and K. Tiefenbacher: Angew. Chem. Int. Ed. 2013, 52, 2.
- Selected for highlight in C&EN: “2013’s Notable Advances” 2013, 91, 15.
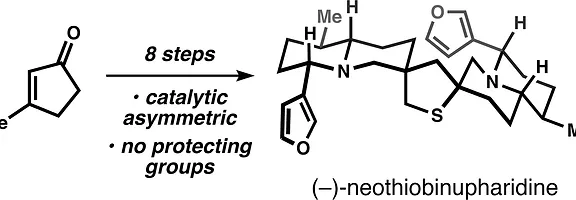
16. Jansen, D. J.; Shenvi, R. A. Synthesis of (–)-Neothiobinupharidine. J. Am. Chem. Soc. 2013, 135, 1209-1212.
2012
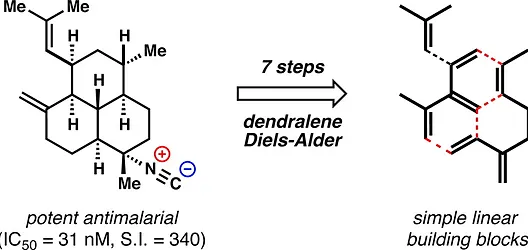
15. Pronin, S. V.; Shenvi, R. A. Synthesis of a Potent Antimalarial Amphilectene. J. Am. Chem. Soc. 2012, 134, 19604-19606.
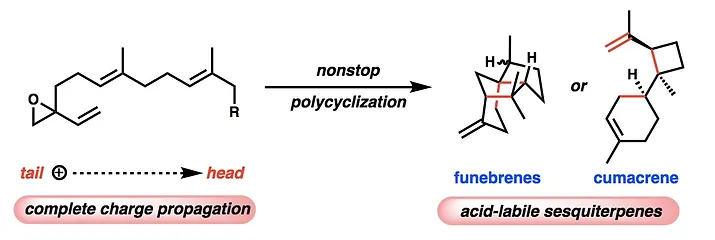
14. Pronin, S. V.; Shenvi, R. A. Synthesis of Highly Strained Terpenes by Nonstop Tail-to-Head Polycyclization. Nature Chem. 2012, 4, 915-920.
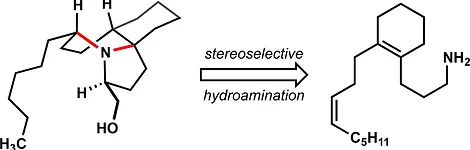
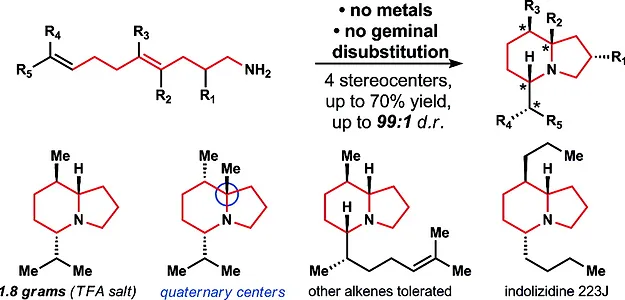
13. Pronin, S. V.; Tabor, M. G.; Jansen, D. J.; Shenvi, R. A. A Stereoselective Hydroamination Transform to Access Polysubstituted Indolizidines. J. Am. Chem. Soc. 2012, 134, 2012-2015.
- In top ten most accessed articles of J. Am. Chem. Soc. for January 2012.
- Highlighted in Nature Chemistry, May 2012.
Postdoctoral, Graduate and Undergraduate
2011
12. Shi, J.; Manolikakes; G. Yeh, C.-H.; Guerrero, C. A.; Shenvi, R. A.; Shigehisa, Hiroki; Baran, P. S. Scalable Synthesis of Cortistatin A and Related Structures. J. Am. Chem. Soc. 2011, 133, 8014-8027.
2010
11. Shenvi, R. A.; Corey, E. J. Synthetic Access to Bent Polycycles by Cation-Pi Cyclization. Org. Lett. 2010, 12, 3548-3551.
2009
10. Shenvi, R. A.; Corey, E. J. A Short and Efficient Synthesis of (–)-7-Methylomuralide, a Potent Proteasome Inhibitor. J. Am. Chem. Soc., 2009, 131, 5746-5747.
9. Shi, J.; Shigehisa, H.; Guerrero, C. A.; Shenvi, R. A.; Li, R. A.; Baran, P. S. Stereodivergent Synthesis of 17-α and 17-β-Aryl Steroids: Application and Biological Evaluation of D-Ring Cortistatin Analogues. Angew. Chem. Int. Ed. 2009, 48, 4328-4331.
8. Shenvi, R. A.; O’Malley, D. P.; Baran, P. S. Chemoselectivity: The Mother of Invention in Total Synthesis. Acc. Chem. Res. 2009, 42, 530-541.
2008
7. Shenvi, R. A. (2008). Pure and Applied Science in the Chemical Syntheses of Marine Alkaloids Chartelline C and Cortistatin A. Ph.D. Thesis, The Scripps Research Institute, 2008.
6. Shenvi, R. A.; Guerrero, C. A.; Shi, J.; Li, C.-C.; Baran, P. S. Synthesis of (+)-Cortistatin A. J. Am. Chem. Soc. 2008, 130, 7241-7243.
2006
5. Baran, P. S.; Shenvi, R. A. Total Synthesis of (±)-Chartelline C. J. Am. Chem. Soc. 2006, 128, 14028-14029.
4. Baran, P. S.; Shenvi, R. A.; Nguyen, S. A. One-Step Synthesis of 4,5-Disubstituted Pyrimidines Using Commercially Available and Inexpensive Reagents. Heterocycles. 2006, 70, 581-586.
2005
3. Baran, P. S.; Shenvi, R. A., Mitsos, C. A. A Remarkable Ring Contraction En Route to the Chartelline Alkaloids. Angew. Chem. Int. Ed. 2005, 44, 3714-3717.
2003
2. Shenvi, R. A.; Funk, R. L. (2003). An approach to the synthesis of the potent antimicrotubule agent ottelione (RPR112378). B.S. Thesis, Pennsylvania State University, 2003.
1. Ababou, A.; Shenvi, R. A.; Desjarlais, J. R. Long-Range Effects on Calcium Binding and Conformational Change in the N-Domain of Calmodulin. Biochemistry 2001, 40, 12719-12726.