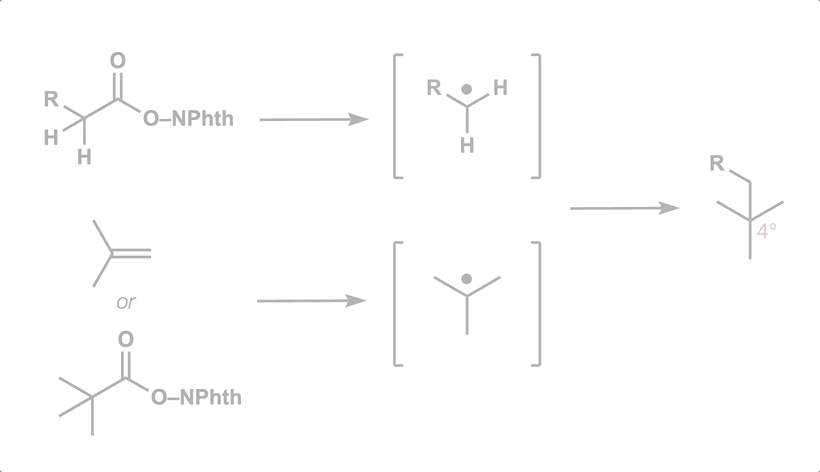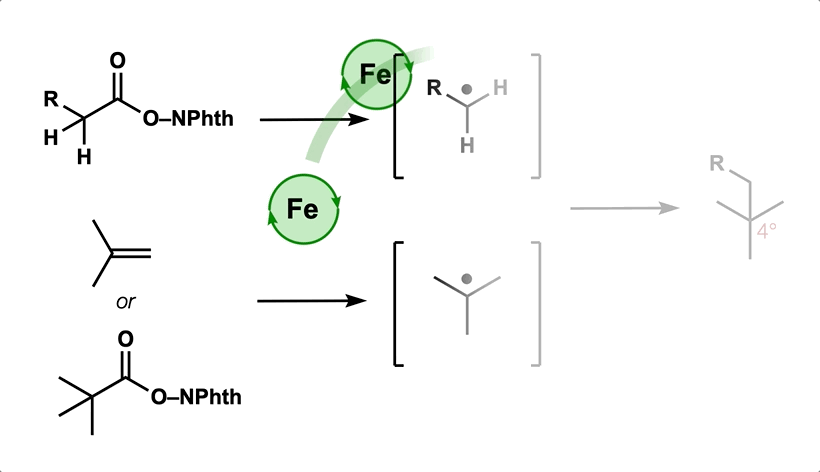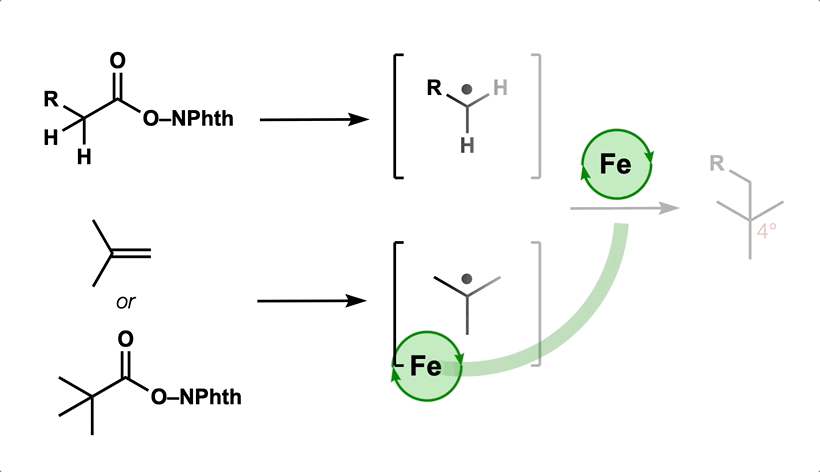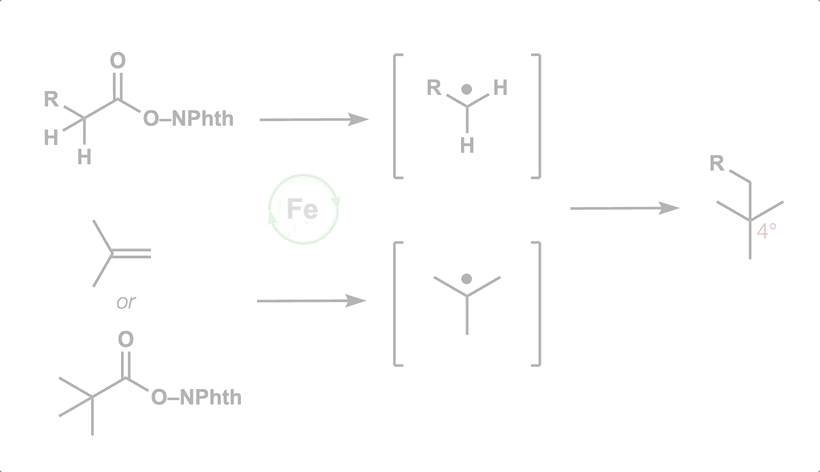
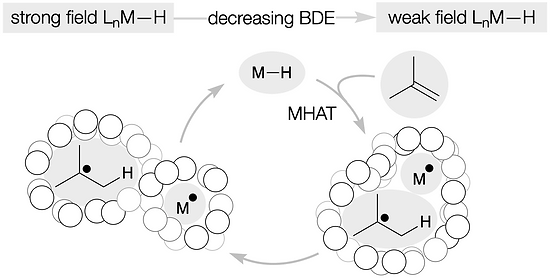
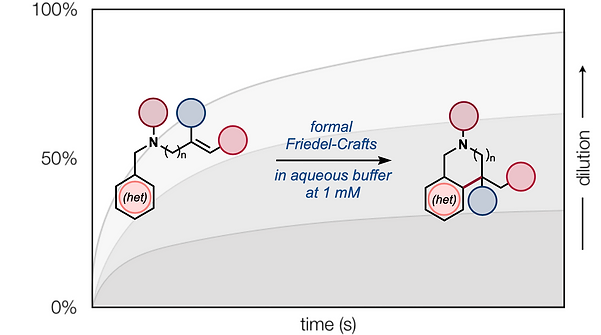
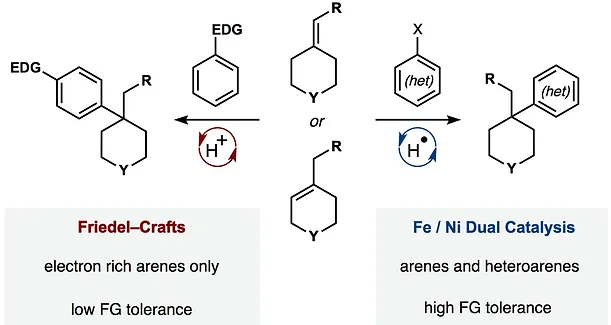
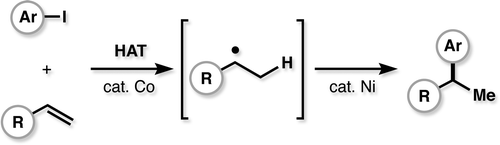
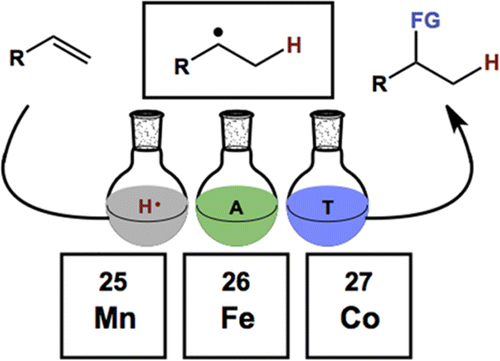
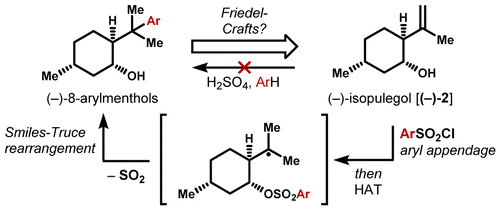
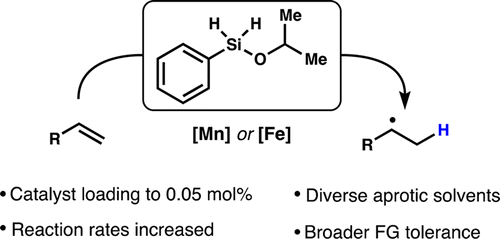
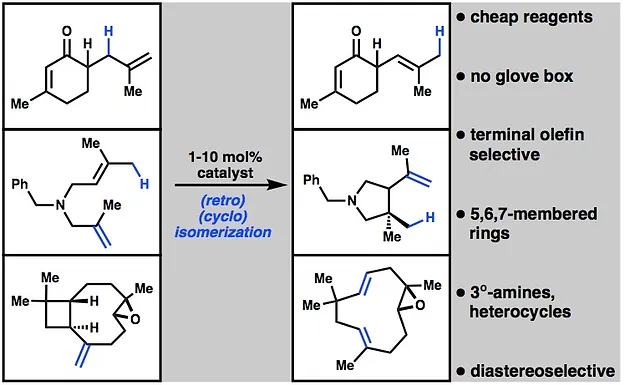
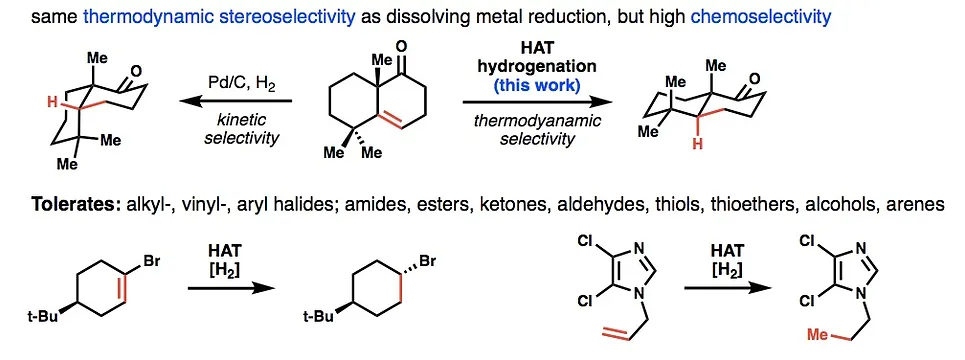
- All
- Ion channel modulators
- Opioids
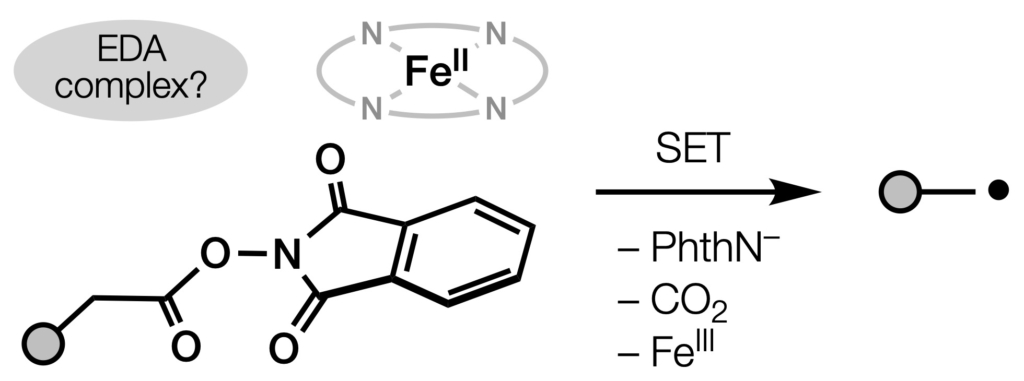
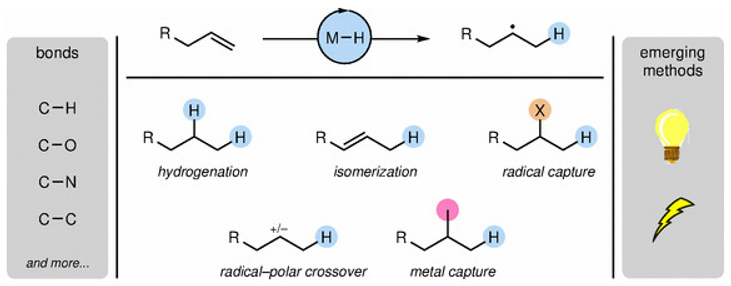
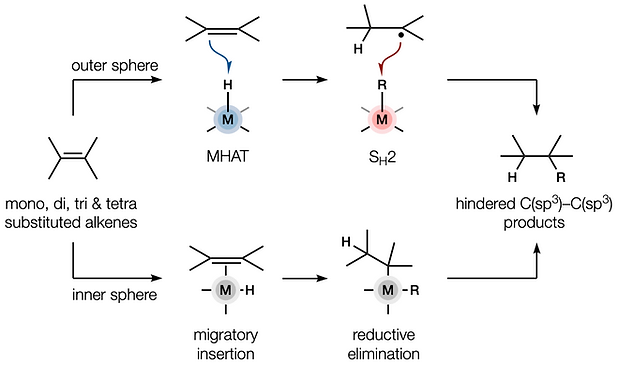
- Metal hydride atom transfer (MHAT)
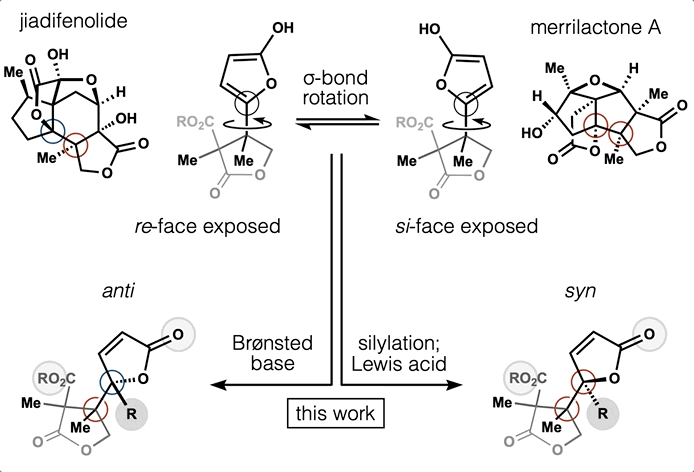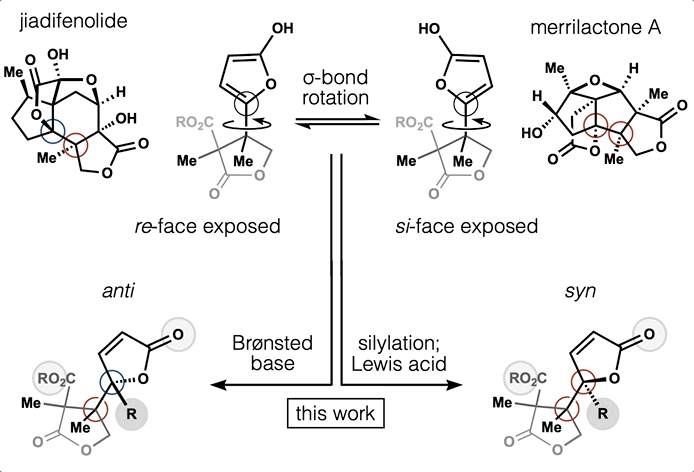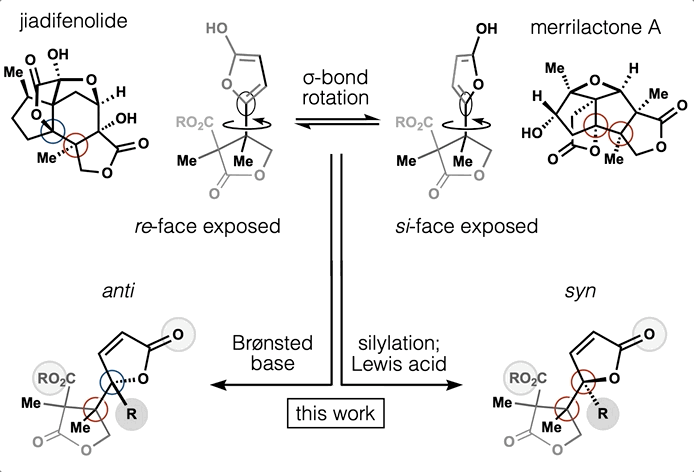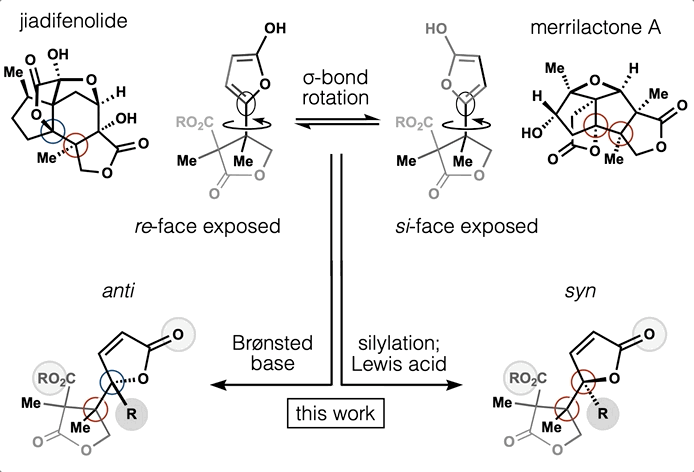
- Synthesis and computation
- Cytotoxins
90. Freeman, S. M.; Landwehr, E. M.; Rojas, J. R.; Tanaka, R.; Bailey, J. B.; Gembicky, M.; Shenvi, R. A.* “Decagram-scale synthesis of GB13, a Galbulimima alkaloid precursor” 2025, ChemRxiv doi: 10.26434/chemrxiv-2025-490r9

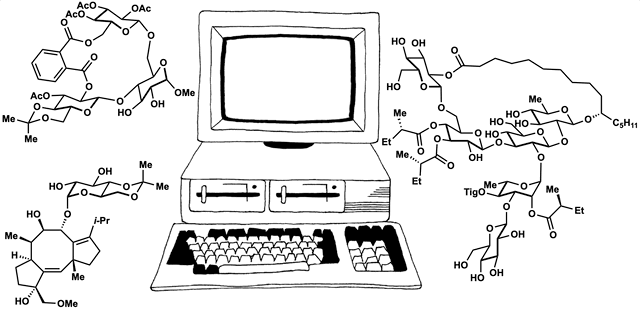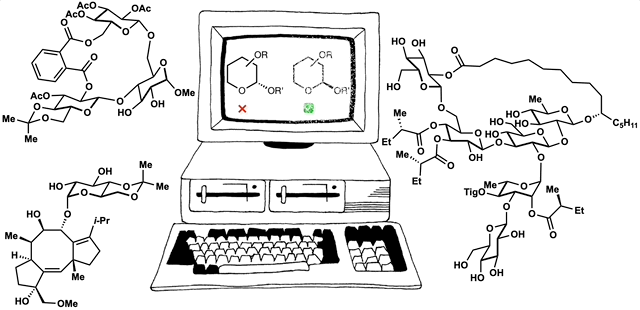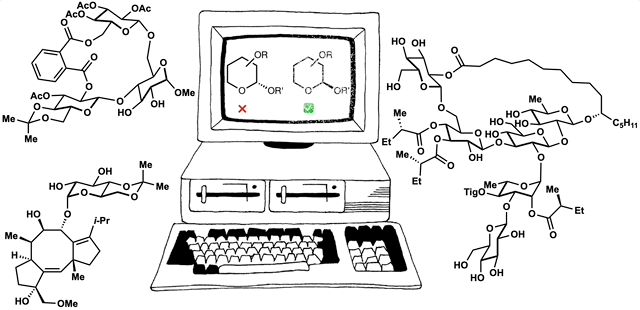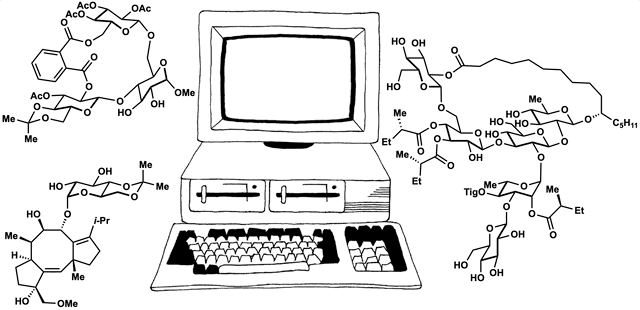
89. Fernandez, S. A.; Snelson, D. W.; Shenvi, R. A. “Digital Duet: Synergism between computation and the synthesis of glycosides” TCI Mail, 2025, accepted.
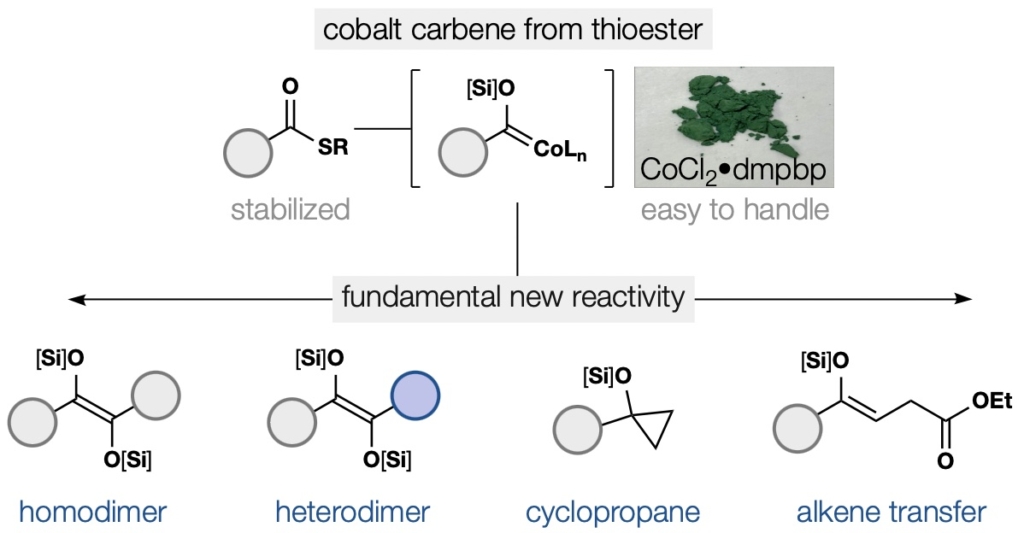
88. Kong, L.;‡ Zong, K.;‡ Guo, J.; Shenvi. R. “Catalytic Acyloin-Type Heterocoupling of Thioesters via a Putative Cobalt Siloxycarbene” 2025, submitted, ChemRxiv doi: 10.26434/chemrxiv-2025-hxpsz
87. Zielke, F.;† Woo, S.;† Kasmali, S.; Volf, A.; Dang, V.; Bailey, J. B.; Gembicky, M.; Bohn, L. M.;* Shenvi. R.* “Bidirectional modification of a Galbulimima alkaloid identifies selective opioid ligands” ACS Central Science, 2025, online. ChemRxiv DOI: 10.26434/chemrxiv-2025-642tw.

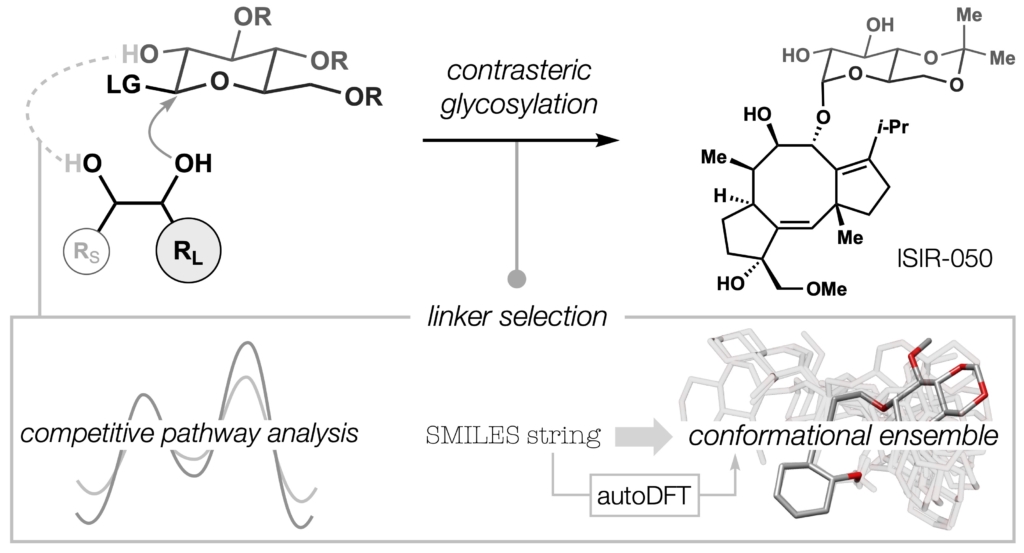
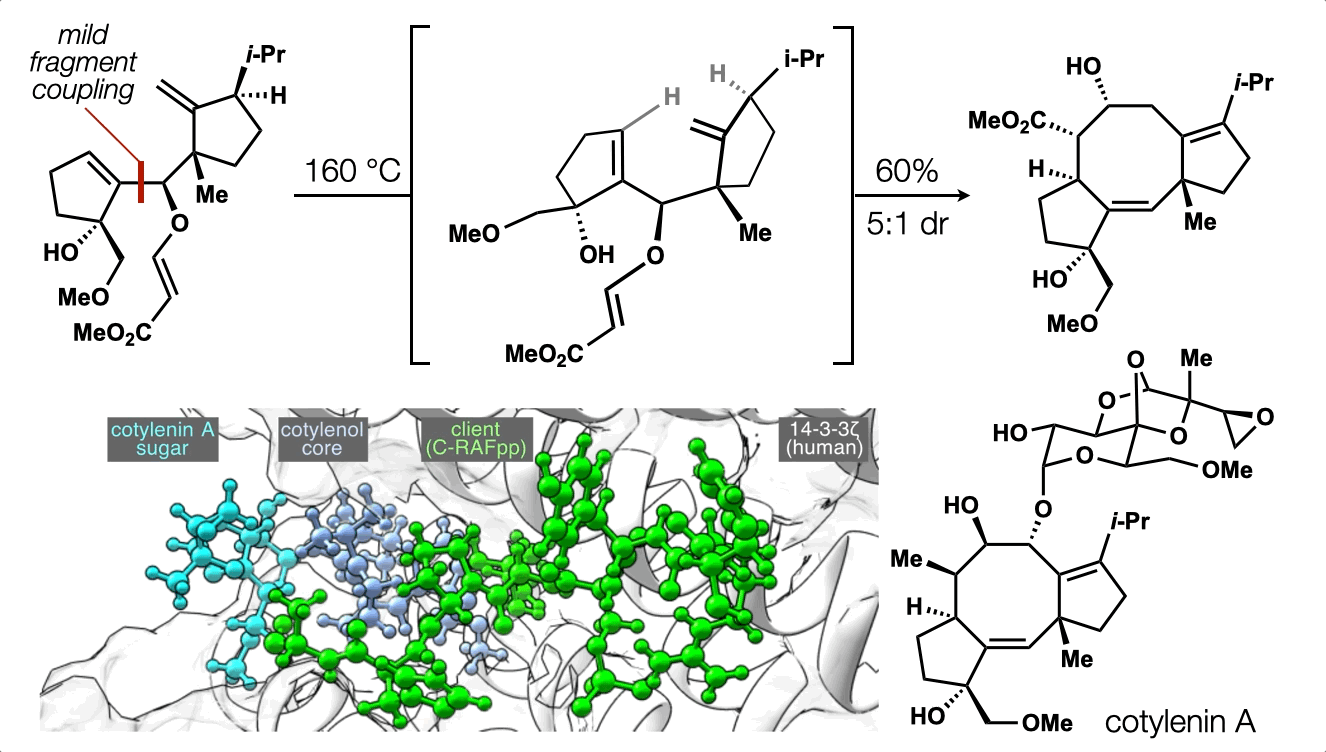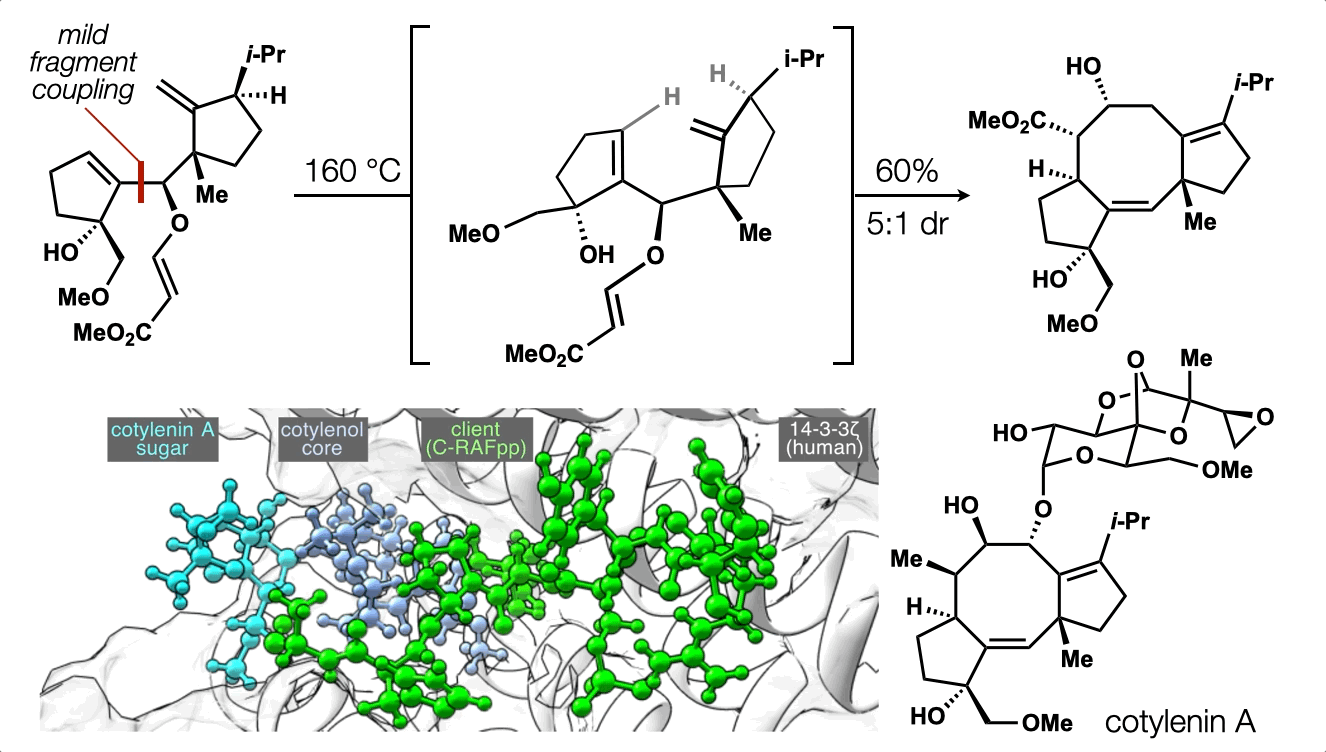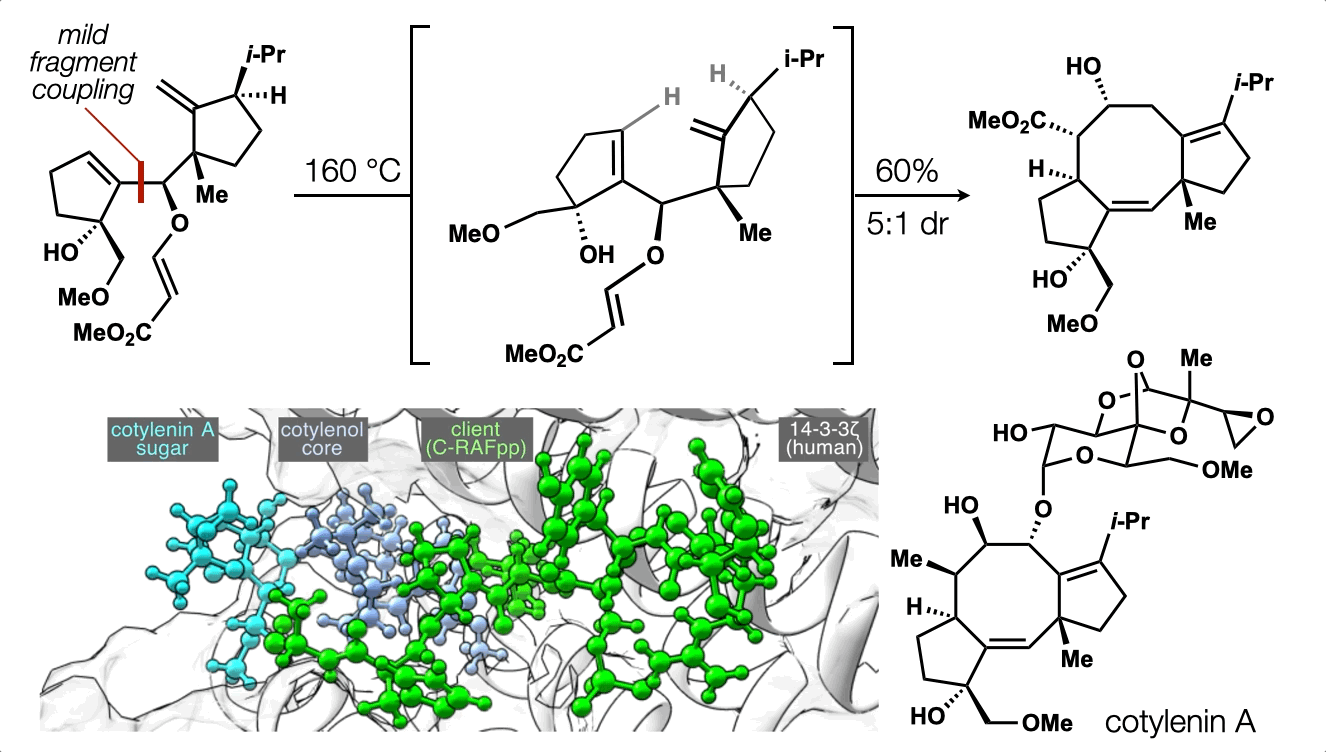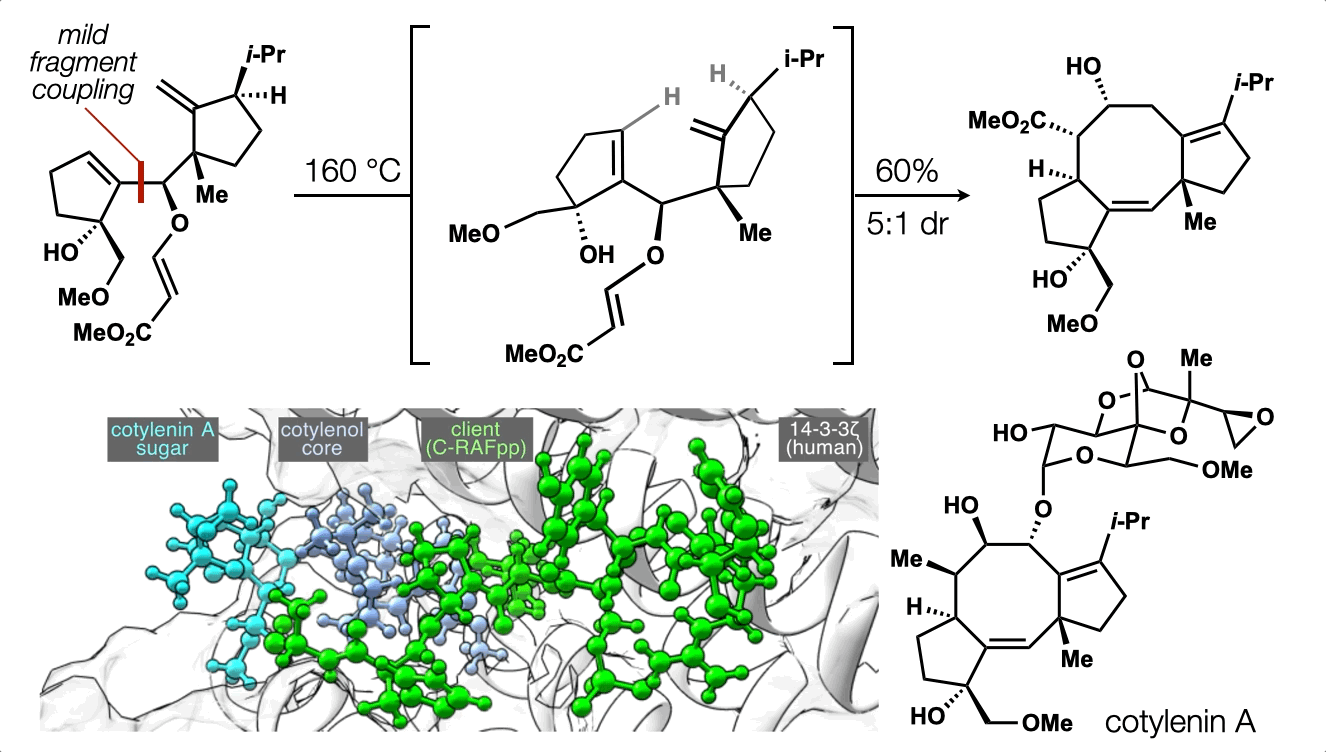
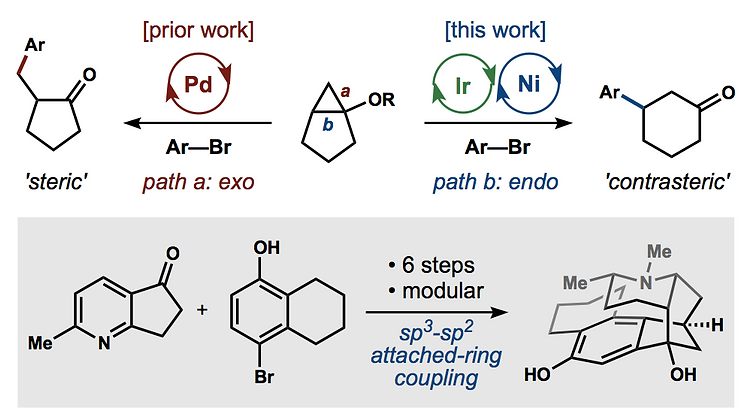
86. Snelson, D.;† Ting, S.;† Shenvi, R. A. “Contrasteric glycosylations of cotylenol and 1,2-diols by virtual linker selection” J. Am. Chem. Soc. 2025, 147, 1327–1333.
85. Li, C.; Shenvi, R. A. “Total synthesis of twenty-five picrotoxanes by virtual library selection” Nature, 2024, accepted. ChemRxiv DOI: 10.26434/chemrxiv-2024-g8j1r
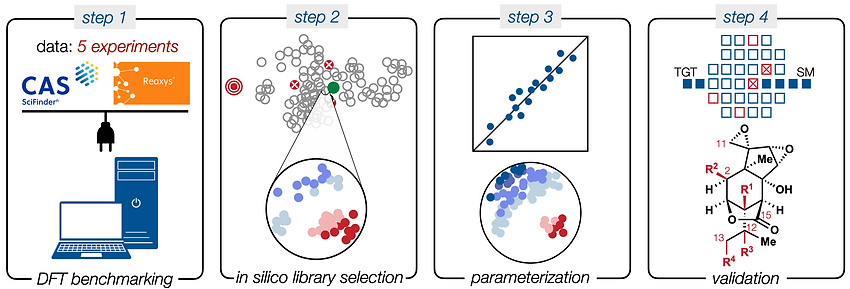
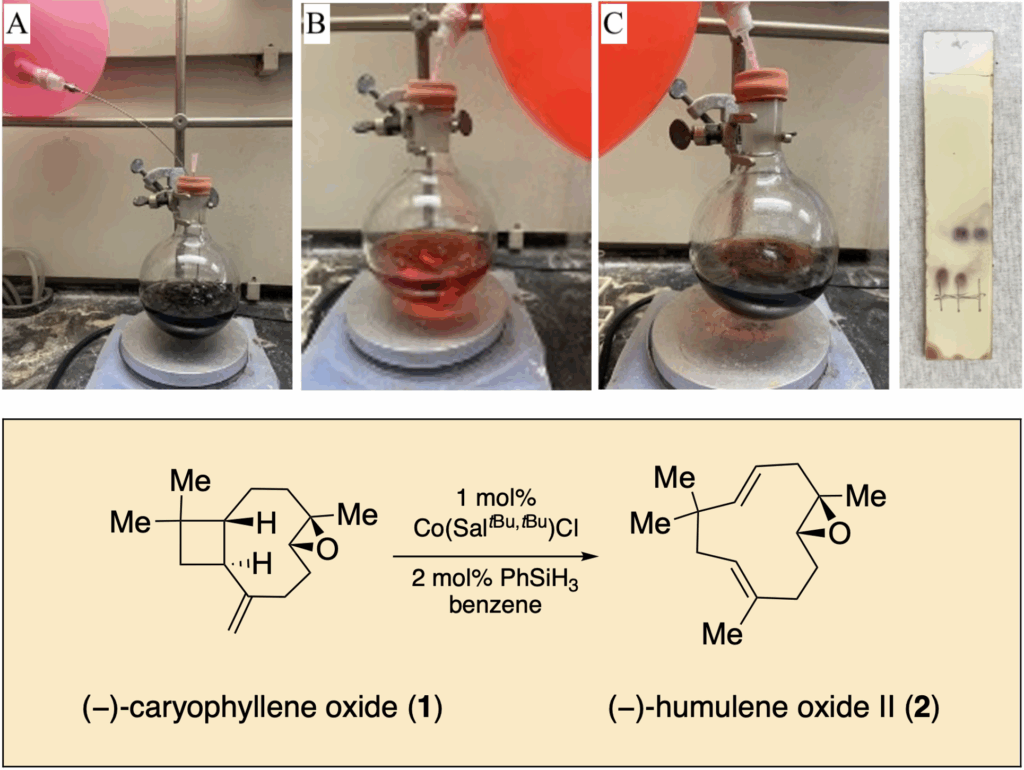
83. Landwehr, E. M.; Shenvi, R. A. “Cobalt-catalyzed radical olefin isomerization: retrocycloisomerization of (–)-caryophyllene oxide to (−)-humulene oxide II” Org. Syn. 2024, 101, 51–60.
[Original protocol in our JACS 2014 16788 paper]
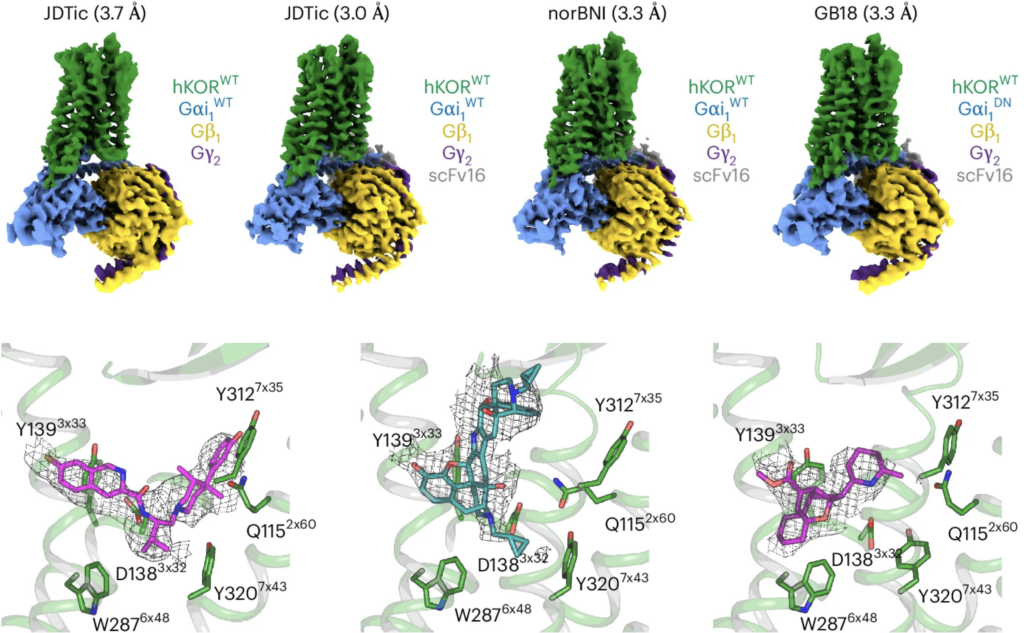
84. Motiwala, Z.; Tyson, A. S.; Styrpejko, D.; Han, G. W.; Khan, S.; Ramos-Gonzalez, N.; Woo, S.; Villers, K.; Landaker, D.; Shenvi, R. A.; Majumdar, S.; Gati, C. “Structural basis of inverse agonism via inactive kappa opioid receptor:G protein complexes” Nature Chem. Bio. 2025, 21, 1046–1057.
82. Clay, K.; Shenvi, R. A. “The original caretakers of salvinorin A” Nature Chem.2024, 16, 1735–1736.
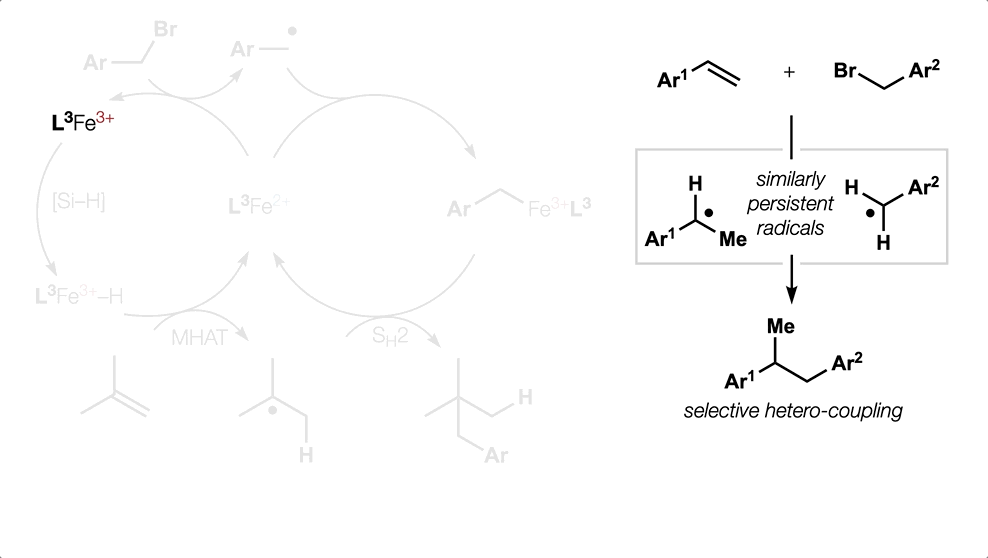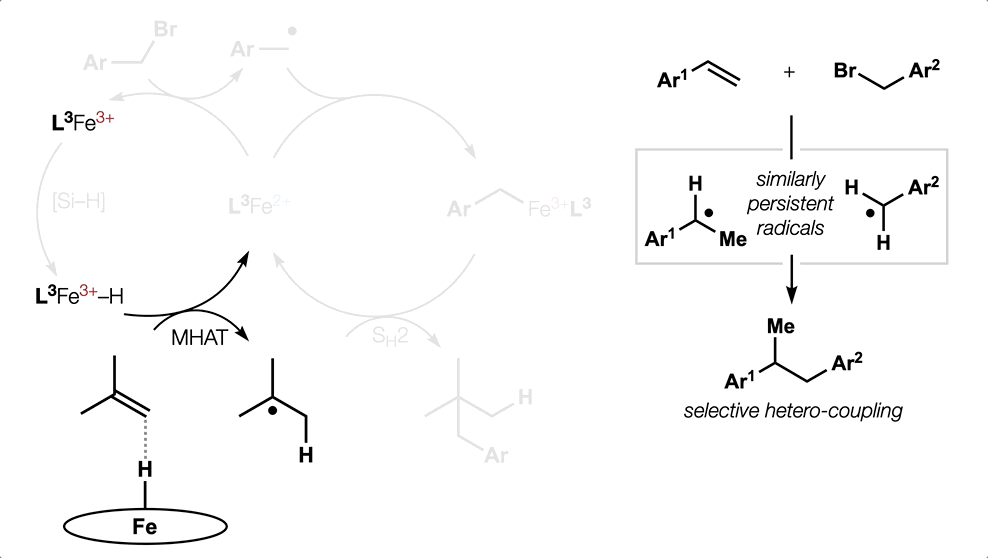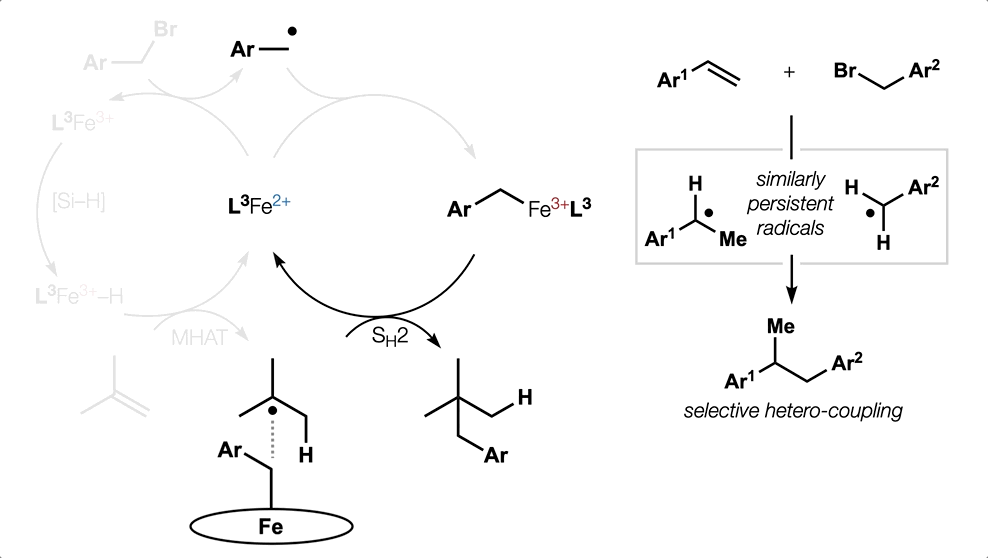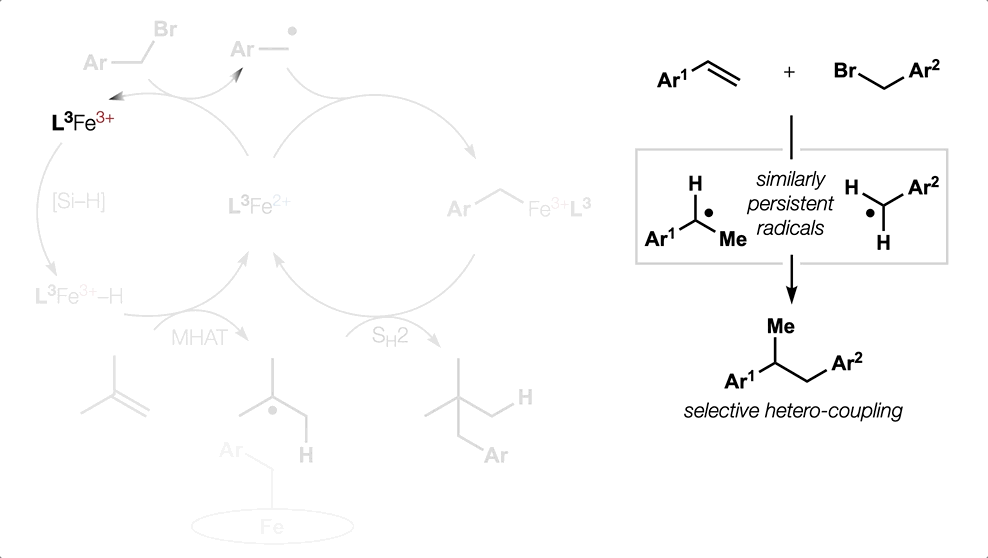
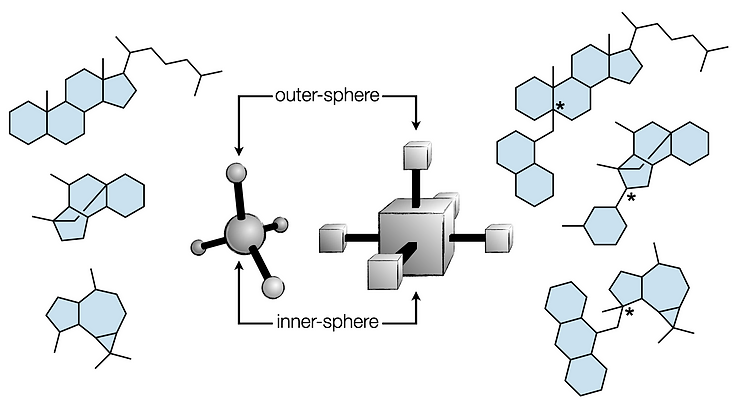
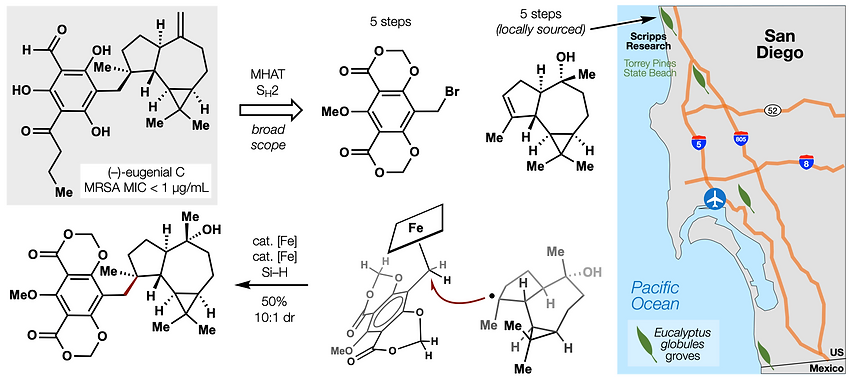
80. Dao, N.; Gan, X.-c.; Shenvi, R. A. “Metal-Hydride C–C Cross-Coupling of Alkenes Through a Double Outer-Sphere Mechanism” [invited] J. Org. Chem. 2024, 89, 16106–16113.
79. Shenvi, R. A. “Natural product synthesis in the 21st century: beyond the mountain top” [invited Perspective] ACS Cent. Sci. 2024, 10, 519–528.

78. Tong, G.; Griffin, S.; Sader, A.; Crowell, A. B.; Beavers, K.; Watson, J.; Buchan, Z.; Chen, S.; Shenvi, R. A. C5 Methylation Confers Accessibility, Stability and Selectivity to Picrotoxinin Nature Commun. 2023, 14, 8308.
ChemRxiv DOI: 10.26434/chemrxiv-2022-0l4nt
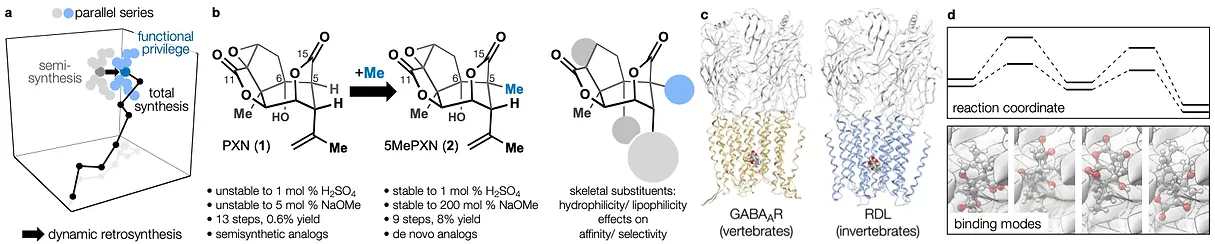
74. Ting, S.;† Snelson, D.;† Huffman, T. R.; Kuroo, A.; Sato, R.; Shenvi, R. A. Synthesis of (–)-cotylenol, a 14-3-3 molecular glue component. J. Am. Chem. Soc. 2023, 145, 20634. †co-first authors
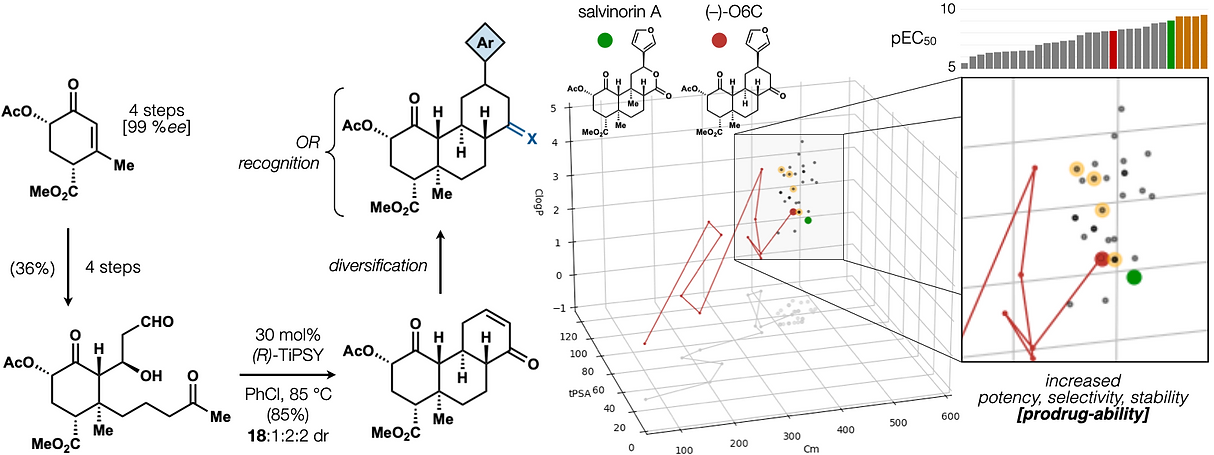
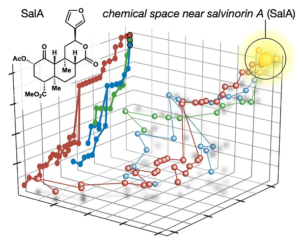
72. Hill, S. J.§; Dao, N.§; Dang, V.; Stahl, E.; Bohn, L. Shenvi, R. A. A route to potent, selective and biased salvinorin chemical space. ACS Cent. Sci., 2023, online. §co-first authors
71. Shenvi, R. A. Early Childhood Care as an Academic: The Slow Burn. Angew. Chem. Int. Ed., 2023, in press [Ihr verborgenes Leben (Their Hidden Lives) Essay Series].

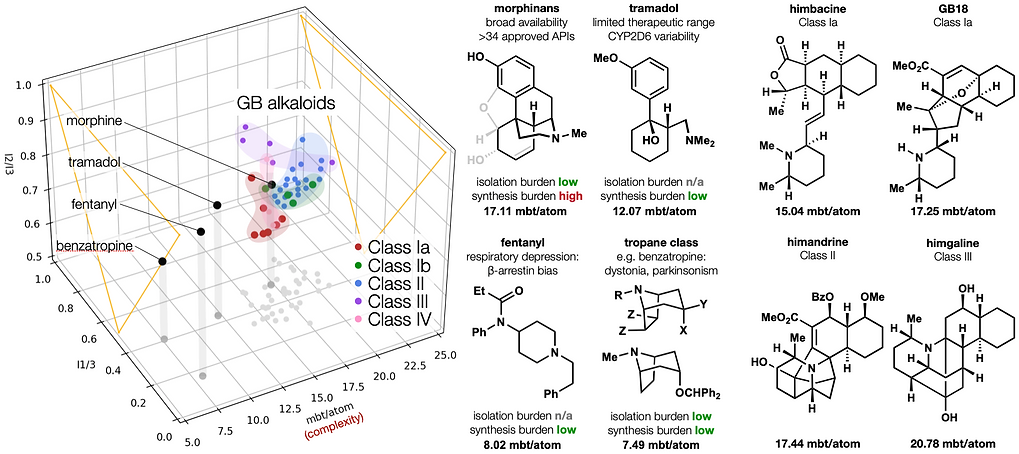
69. Woo, S.; Landwehr, E.; Shenvi, R. A. Synthesis of psychotropic alkaloids from Galbulimima Tetrahedron 2022, 126, 133064. [In celebration of the 65th anniversary of Tetrahedron Publications]
68. Shevick, S. L.; Freeman, S.; Tong, G.; Russo, R. J.; Bohn, L. M.; Shenvi, R. A. Asymmetric syntheses of (+)- and (–)-collybolide enable re-evaluation of kappa-opioid receptor agonism ACS Cent. Sci., 2022, 8, 948–954. DOI: https://doi.org/10.1021/acscentsci.2c00442.
ChemRxiv DOI: 10.26434/chemrxiv-2021-rtxqf
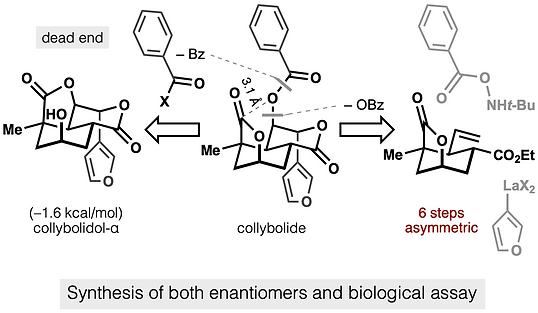
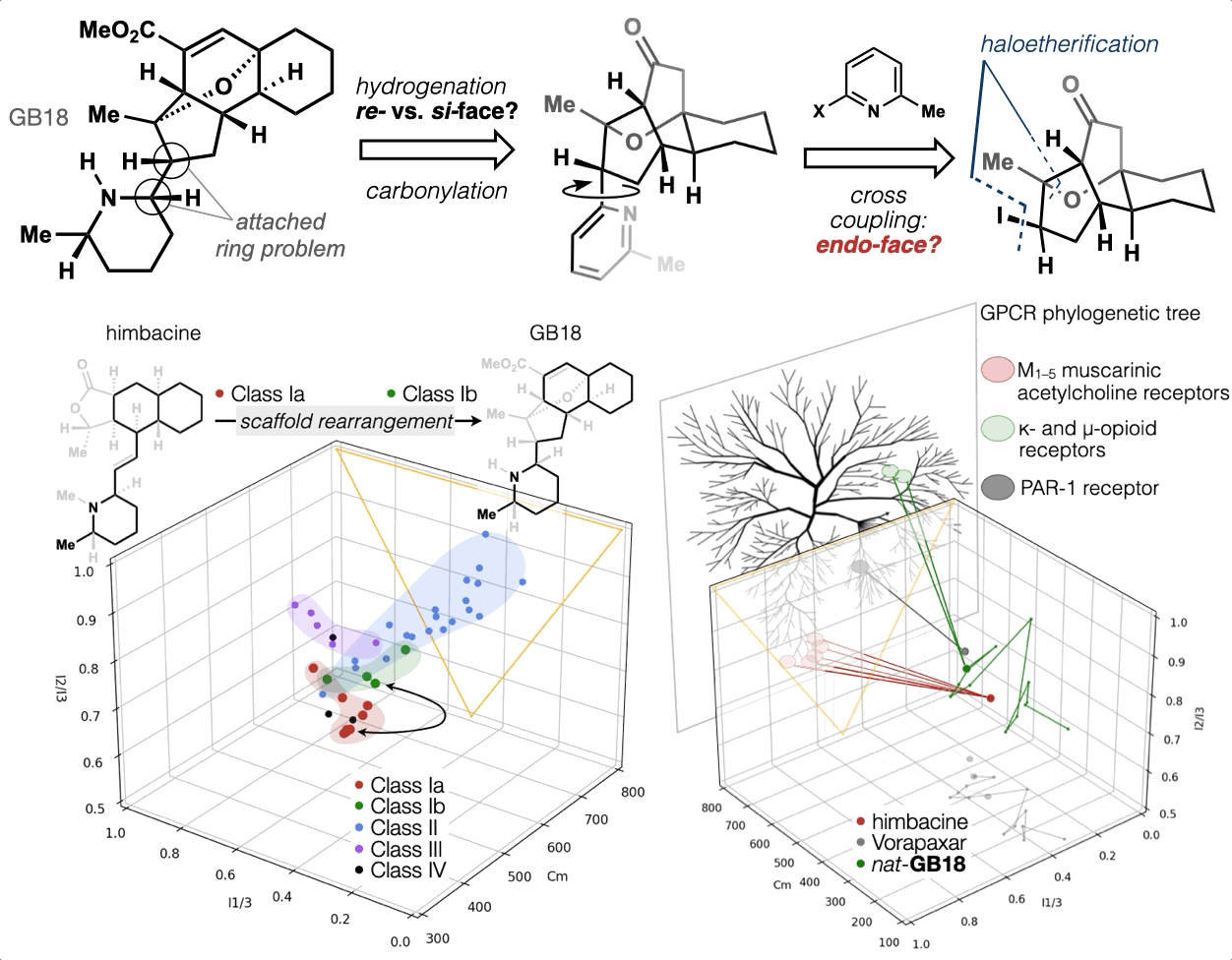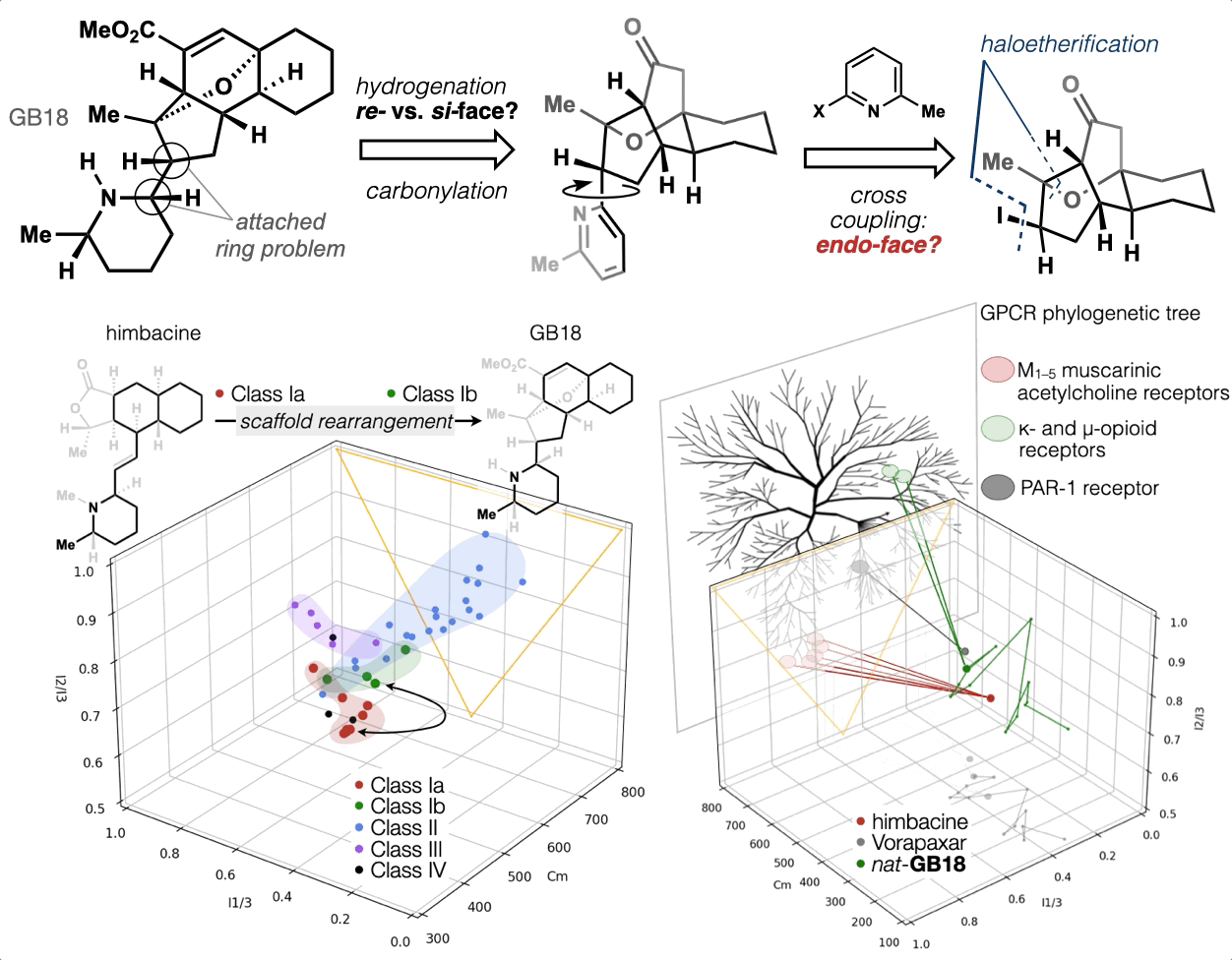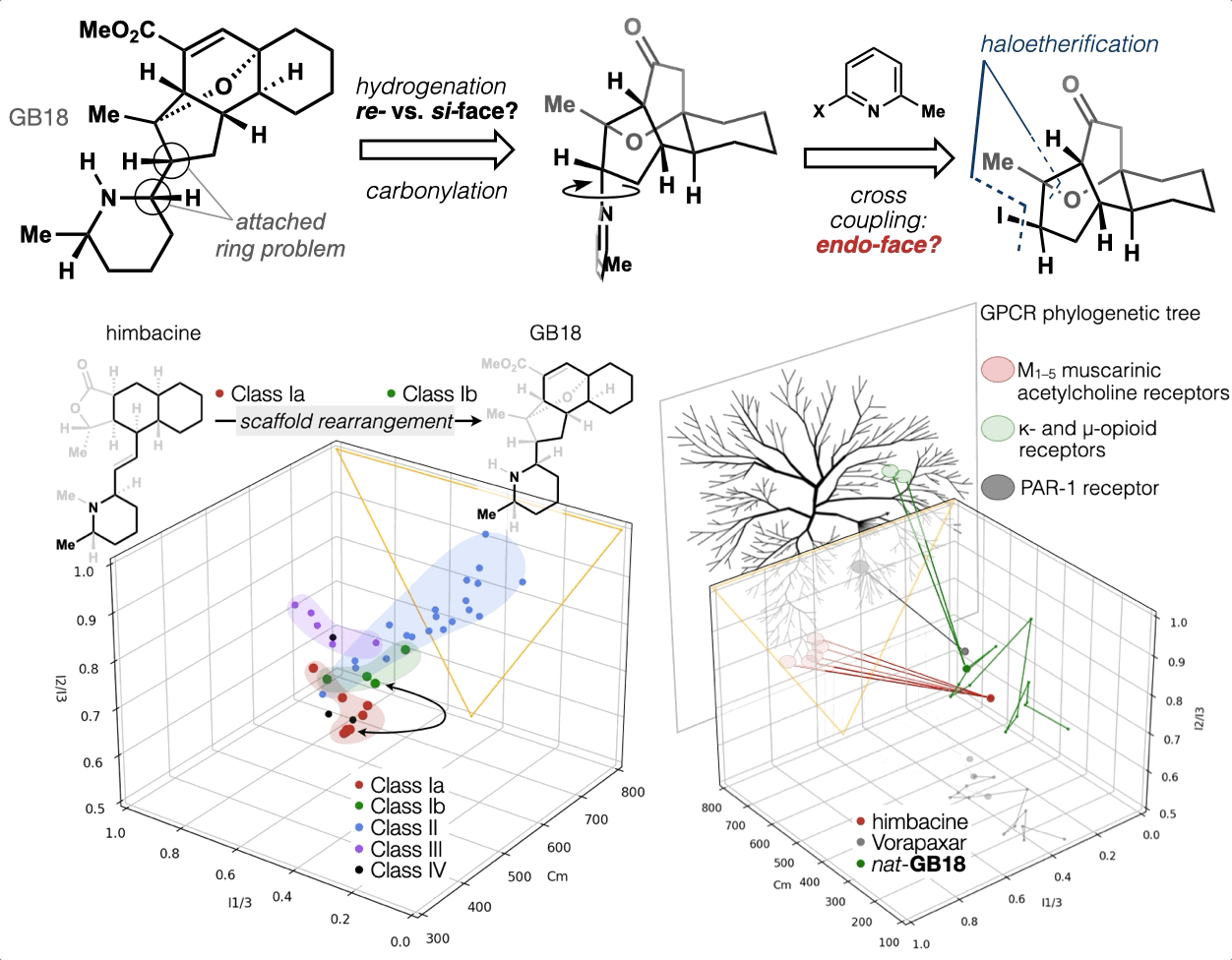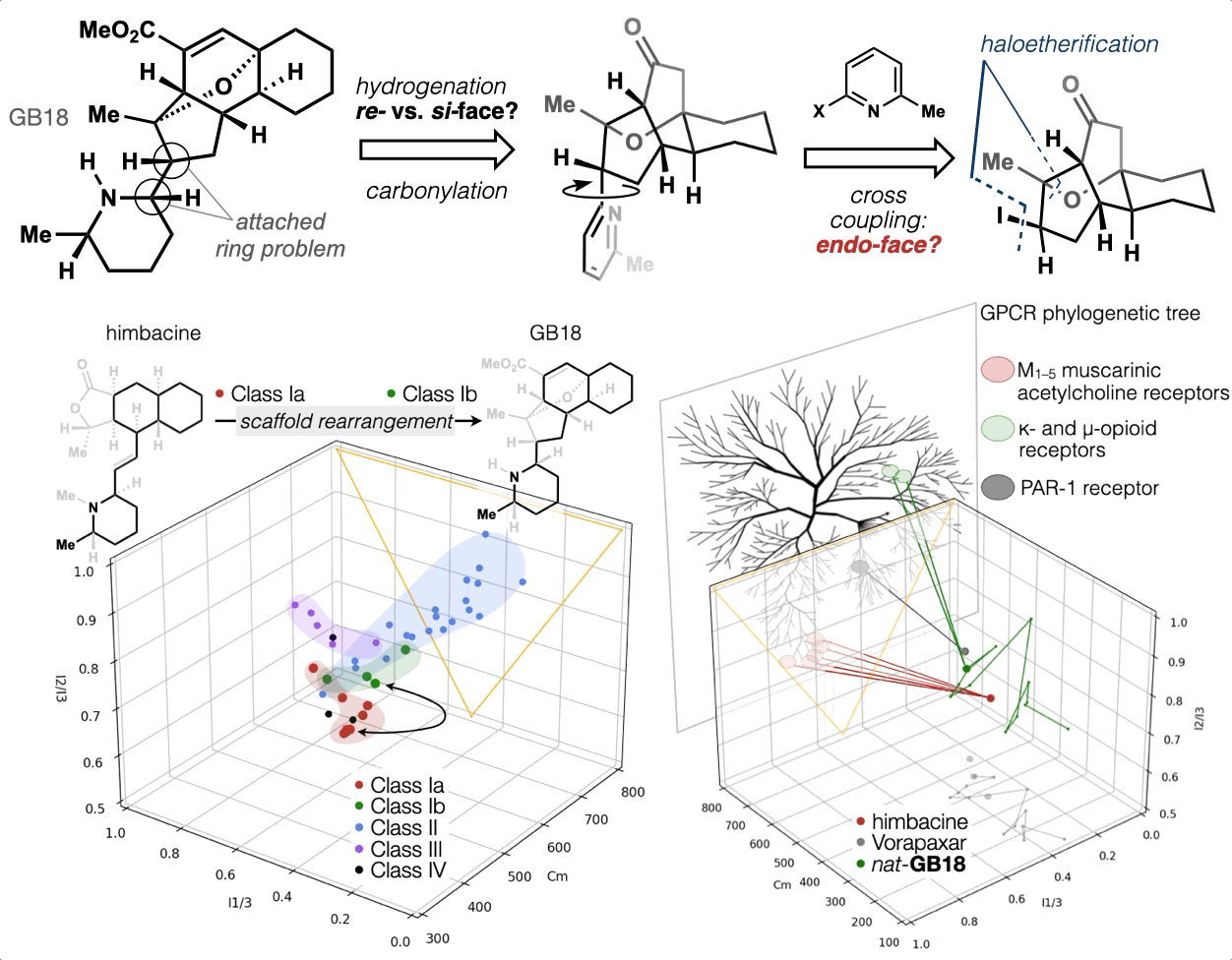
67. Woo, S.; Shenvi, R. A. Synthesis and target annotation of GB18. Nature, 2022, DOI: 10.1038/s41586-022-04840-9.
ChemRxiv DOI: 10.26434/chemrxiv-2022-cs8v5
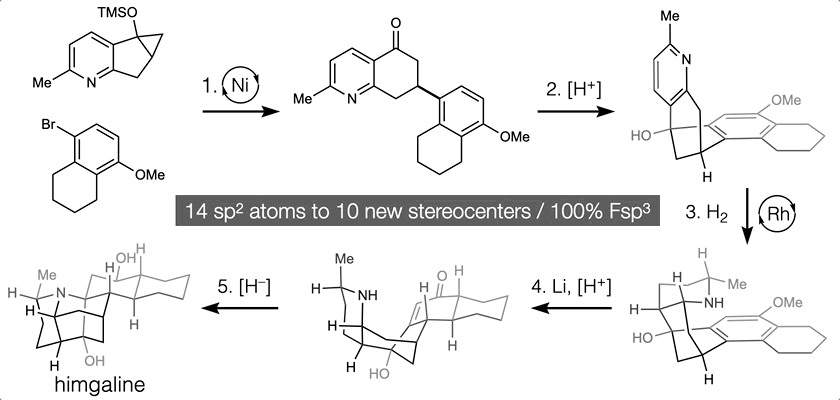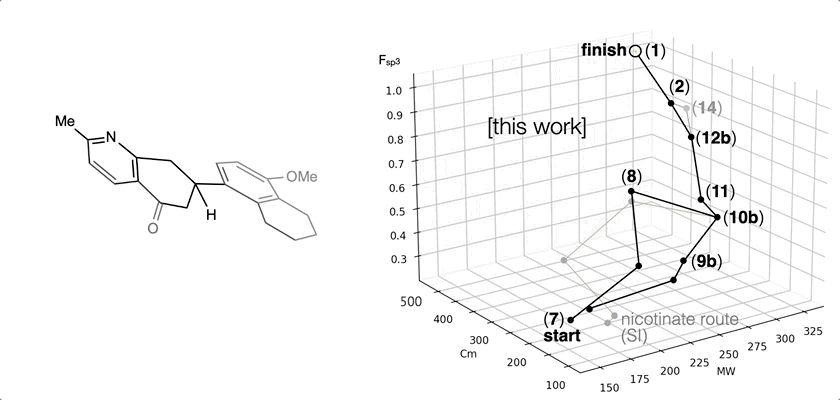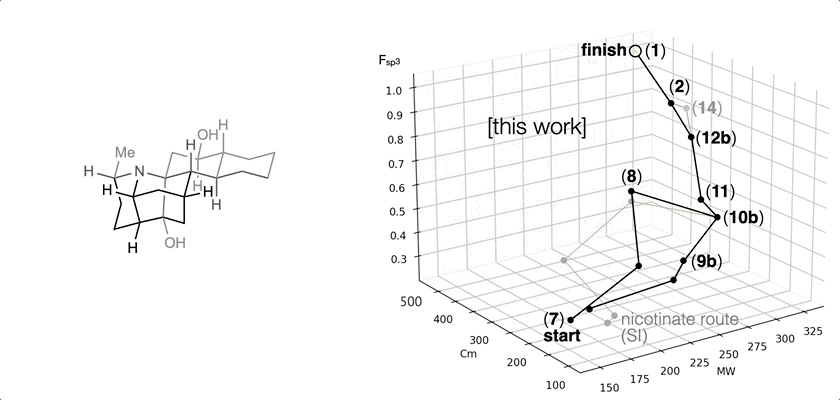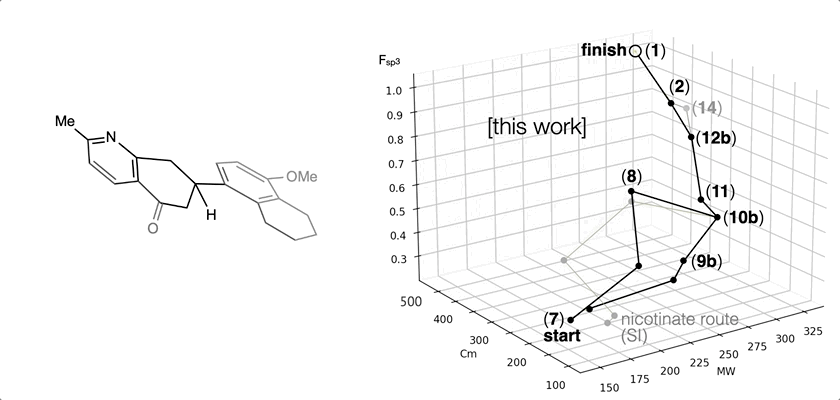
66. Landwehr, E. M.; Baker, M. A.; Oguma, T.; Burdge, H. E.; Kawajiri, T.; Shenvi, R. A. Concise syntheses of GB22, GB13 and himgaline by cross-coupling and complete reduction. Science, 2022, 375, 1270–1274.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
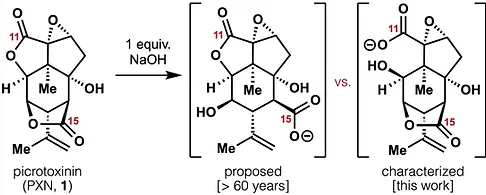
64. Tong, G.; Shenvi, R. A. Revision of the unstable picrotoxinin hydrolysis product Angew. Chem. Int. Ed. 2021, 60, 19113.
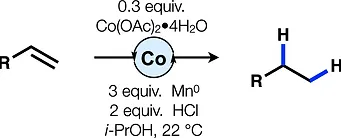
63. van der Puyl, V.; McCourt, R. O.; Shenvi, R. A. Cobalt-catalyzed alkene hydrogenation by reductive turnover Tetrahedron Lett. 2021, 72, 153047. On the occasion of the 2020 Tetrahedron Prize to Dale Boger.
62. Woo, S.; Shenvi, R. A. Natural Product Synthesis through the Lens of Informatics Acc. Chem. Res. 2021, 54, 1157–1167.
61. Tong, G.; Baker, M. A.; Shenvi, R. A. Change the channel: CysLoop receptor antagonists from Nature. Pest Manag. Sci. 2020, 77, 3650–3662. [Special Issue honoring Tom Sparks for the Kenneth Spencer Award]
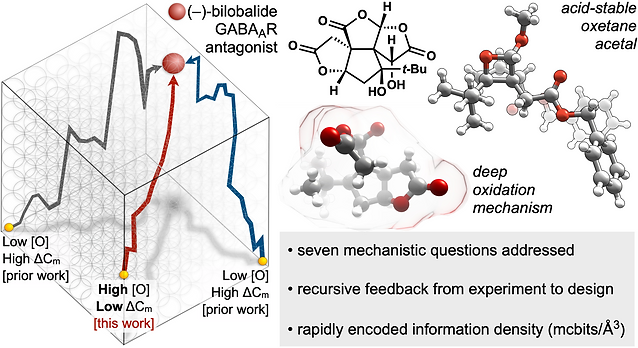
59. Demoret, R. M.; Baker, M. A.; Ohtawa, M.; Chen, S.; Lam, C.-C.; Khom, S.; Roberto, M.; Forli, S.; Houk, K.; Shenvi, R. A. Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide. J. Am. Chem. Soc. 2020, 142, 18599–18618.ChemRxiv DOI: 10.26434/ chemrxiv.12132939.v2
58. Hill, S. J.; Brion, A. U. C. M.; Shenvi, R. A. Chemical Syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020, 37, 1478–1496.

57. Crossley, S. W. M.; Tong, G.; Lambrecht, M. J.; Burdge, H. E.; Shenvi, R. A. Total Synthesis of (–)-Picrotoxinin J. Am. Chem. Soc. 2020, 142, 11376–11381.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
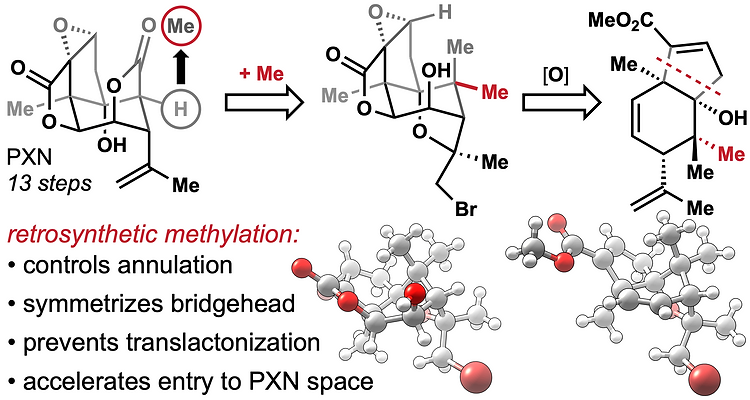
55. Burdge, H. E.; Oguma, T.; Kawajiri, T.; Shenvi, R. A. Concise Synthesis of GB22 by Endo-Selective Siloxycyclopropane Arylation ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1 .
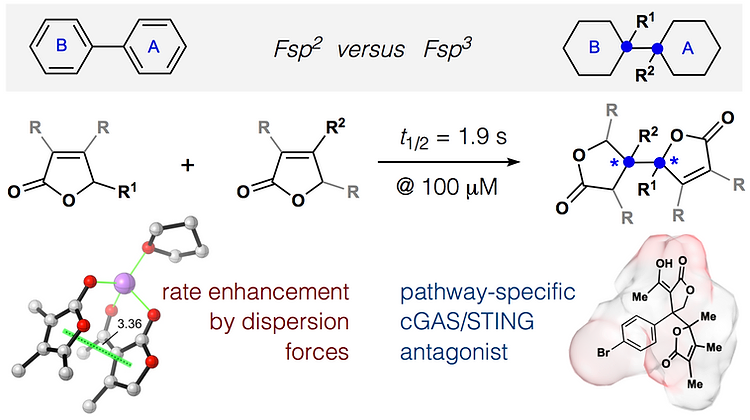
54. Huffman, B. J.; Chen, S.; Schwarz, J. L; Plata, R. E.; Chin, E.; Houk, K. N.; Lairson, L. L.; Shenvi, R. A. Electronic Complementarity Permits Hindered Butenolide Heterodimerization and Discovery of Novel cGAS/STING Pathway Antagonists, Nature Chem. 2020, 12, 310–317.
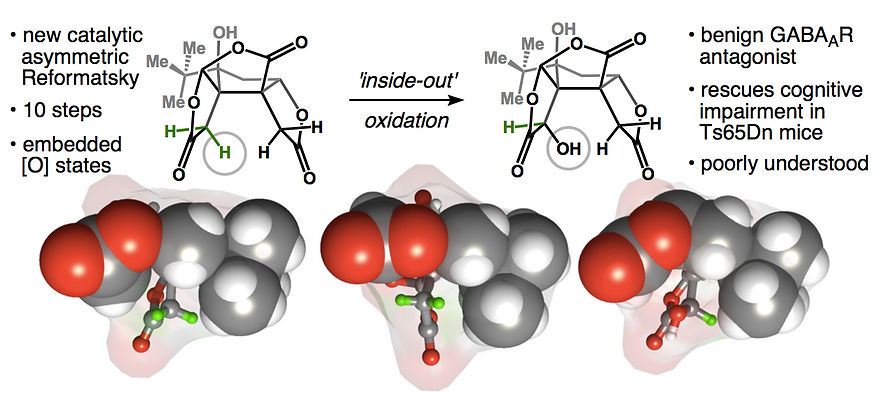
51. Baker, M.; Demoret, R.; Ohtawa, M.; Shenvi, R. A. Concise Asymmetric Synthesis of (–)-Bilobalide Nature 2019, 575, 643–646. DOI: 10.1038/s41586-019-1690-5. ChemRxiv DOI: 10.26434/chemrxiv.8202053.v1 Featured in Org. Chem. Highlights
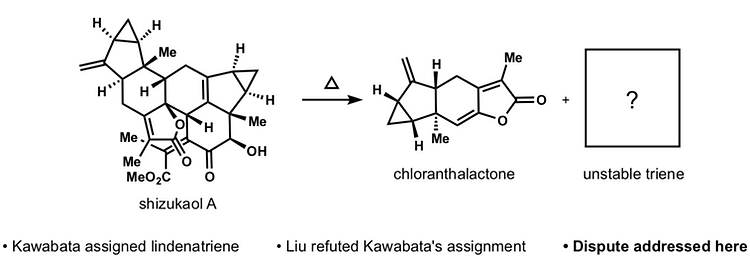
49. Martinez, R.; Burdge, H.; Shenvi, R. A. Reanalysis of Lindenatriene, a Building Block for the Synthesis of Lindenane Oligomers Tetrahedron 2019, 75, 3140-3144. [invited manuscript for Ryan’s Tetrahedron Young Investigator Award 2019]
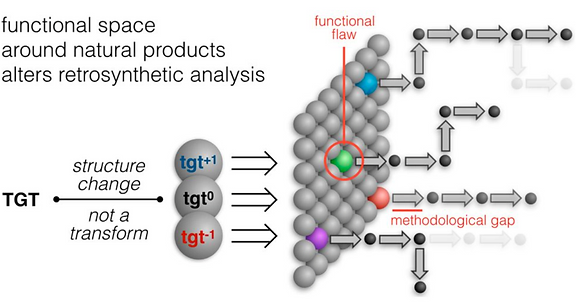
48. Huffman, B.; Shenvi, R.A. Natural Products in the ‘Marketplace’: Interfacing Synthesis and Biology J. Am. Chem. Soc. 2019, 141, 3332-3346.
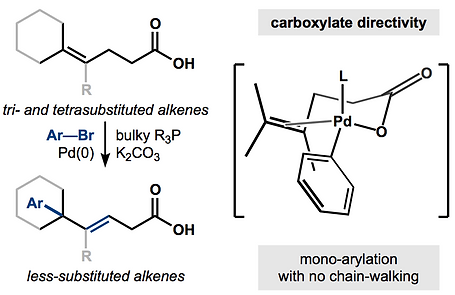
47. Huffman, T.; Wu, Y.; Emmerich, A.; Shenvi, R.A. Intermolecular Heck Coupling with Hindered Alkenes Directed by Potassium Carboxylates Angew. Chem. Int. Ed. 2019, 58, 2371-2376.
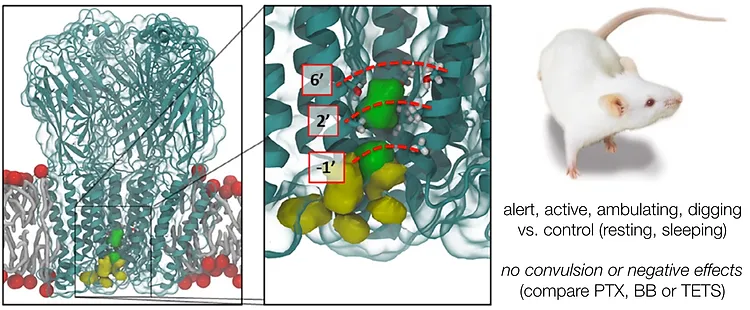
42. Jeffrey M. Witkin, Ryan A. Shenvi, Xia Li, Scott D. Gleason, Julie Weiss, Denise Morrow, John T. Catow, Mark Wakulchik, Masaki Ohtawa, Hai-Hua Lu, Michael D. Martinez, Jeffrey M. Schkeryantz, Timothy S. Carpenter, Felice C. Lightstone, Rok Cerne Pharmacological characterization of the neurotrophic sesquiterpene jiadifenolide reveals a non-convulsant signature and potential for progression in neurodegenerative disease studies. Biochem. Pharm. 2018, 155, 61–70.
DOI: 10.1016/j.bcp.2018.06.022
41. Lambrecht, M.; Kelly, J. W.; Shenvi, R. A. Mechanism of Action of the Cytotoxic Asmarine Alkaloids. ACS Chem. Bio. 2018, 13, 1299–1306. DOI: 10.1021/acschembio.8b00096
40. Roach, J. J.; Shenvi, R. A. A Review of Salvinorin Analogs and their Kappa-Opioid Receptor Activity. Bioorg. Med. Chem. Lett. 2018, 28, 1436–1445. DOI: 10.1016/j.bmcl.2018.03.029
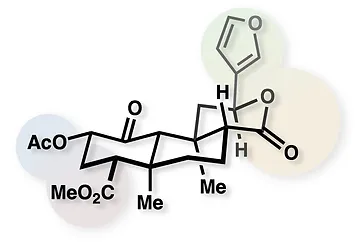
39. Hirasawa, S.; Cho, M.; Brust, T. F.; Roach, J. J.; Bohn, L. M.; Shenvi, R. A. O6C-20-nor-SalA is a stable and potent KOR agonist. Bioorg. Med. Chem. Lett. 2018, 28, 2770–2722. Special Issue dedicated to Dale Boger.
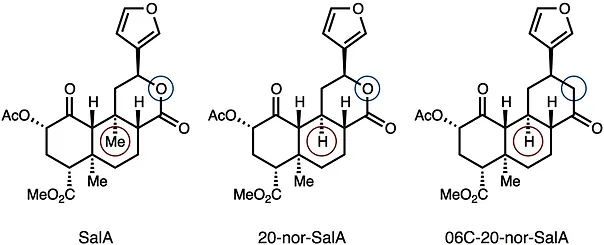
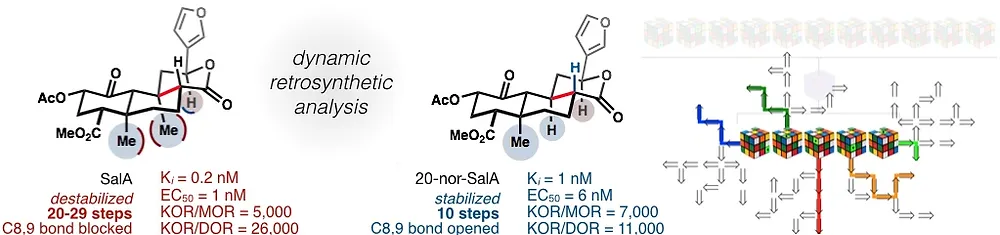
38. Dynamic Strategic Bond Analysis Yields a 10-step Synthesis of 20-nor-SalA, a Potent Κ-OR Agonist
38. Roach, J. J.; Sasano, Y.; Schmid, C. L.; Zaidi, S.; Katrich, V.; Stevens, R. C.; Bohn, L. M.; Shenvi, R. A. Dynamic Strategic Bond Analysis Yields a 10-step Synthesis of 20-nor-SalA, a Potent Κ-OR Agonist. A(CS)2 2017, 3, 1329–1336.
ChemRxiv DOI: 10.26434/chemrxiv.5318188
- #1 Most Viewed Article on ChemRxiv, Aug-Dec 2017.
- Highlighted by Tien Ngyuen in C&EN, Sept. 1 2017.
- See article on SlashGear by Brittany Rosen, Sept. 11, 2017
- See article on Gizmodo by Ryan Mandalbaum, Sept. 12, 2017
- See article on Geek by Daniel Starkey, Sept. 12, 2017
- See article on Wired by Matthew Simon, Jan. 8, 2018
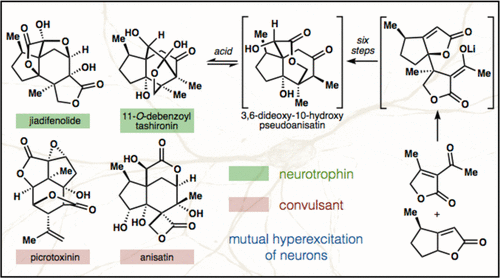
37. Ohtawa, M.; Krambis, M. J.; Cerne, R.; Schkeryantz, J.; Witkin, J. M.; Shenvi, R. A. Synthesis of (–)-11-O-Debenzoyltashironin: Neurotrophic Sesquiterpenes Cause Hyperexcitation. J. Am. Chem. Soc. 2017, 139, 9637-9644.
Featured in Org. Chem. Highlights
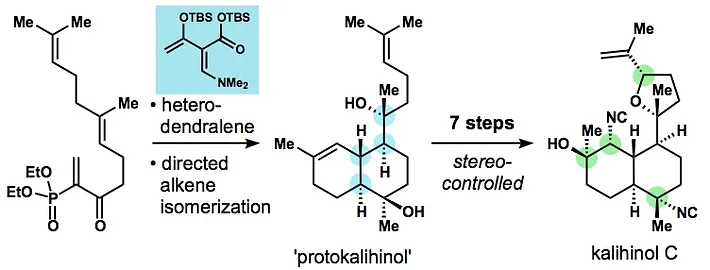
36. Reiher, C. A.; Shenvi, R. A. Stereocontrolled Synthesis of Kalihinol C. J. Am. Chem. Soc. 2017, 139, 3647-3650.
34. Shenvi, R. A. And You, of Tender Years. Chem. 2016, 1, 331.
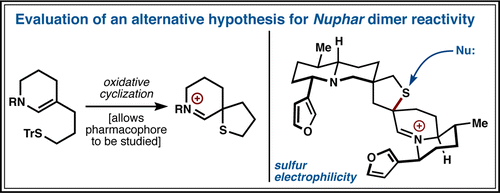
32. Tada, N.; Jansen, D. J.; Mower, M. P.; Blewett, M. M.; Umotoy, J. C.; Cravatt, B. F.; Wolan, D. W.; Shenvi, R. A. Synthesis and Sulfur Electrophilicity of the Nuphar Thiaspirane Pharmacophore. ACS Cent. Sci. 2016, 2, 401-408.
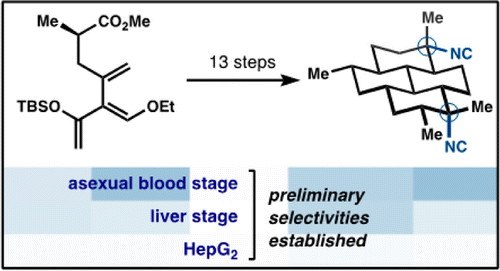
31. Lu, H.-H.; Pronin, S. V.; Antonova-Koch, Y.; Meister, S.; Winzeler, E. A.; Shenvi, R. A. Synthesis of (+)-7,20-Diisocyanoadociane and Liver-Stage Antiplasmodial Activity of the Isocyanoterpene Class. J. Am. Chem. Soc. 2016, 138, 7268-7271.
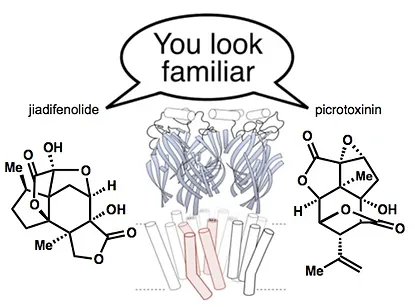
28. Shenvi, R. A. Neurite Outgrowth Enhancement by Jiadifenolide: Possible Targets. Nat. Prod. Rep. 2016, 33, 535-539.
26. Wan, K. K.; Shenvi, R. A. Conjuring a Supernatural Product – Delmarine. SynLett (invited Accounts). 2016, 27, 1145-1164.
25. Tabor, M. G.; Shenvi, R. A. Synthesis of Lepadiformine Using a Hydroamination Transform. Org. Lett. 2015, 17, 5776.
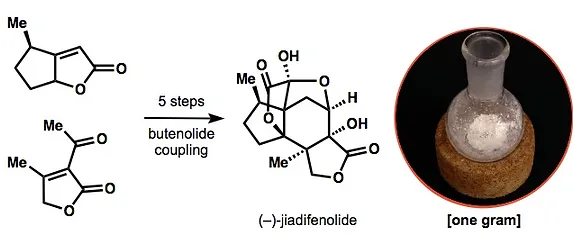
24. Lu, H.-H.; Martinez, M. D.; Shenvi, R. A. Eight-Step, Gram-Scale Synthesis of (–)-Jiadifenolide. Nature Chem. 2015, 7, 604-607.
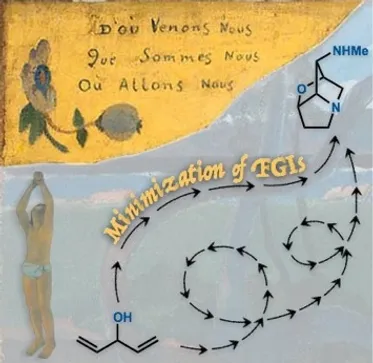
23. Crossley, S. W. M.; Shenvi, R. A. A Longitudinal Study of Alkaloid Synthesis Reveals Functional Group Interconversions (FGIs) as Bad Actors. Chem. Rev. 2015, 115, 9465-9531.
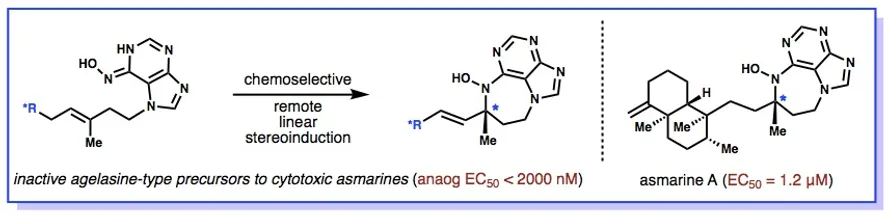
22. Wan, K. K.; Iwasaki, K.; Umotoy, J. C.; Wolan, D.; Shenvi, R. A. Nitrosopurines en route to Potent Asmarine Cytotoxins. Angew.Chem. Int. Ed. 2015, 127, 2440-2445.
21. Shenvi, R. A.; Schnermann, M. J. Syntheses and Biological Studies of Marine Terpenoids Derived from Inorganic Cyanide. Nat. Prod. Rep. 2015, 32, 543-577.
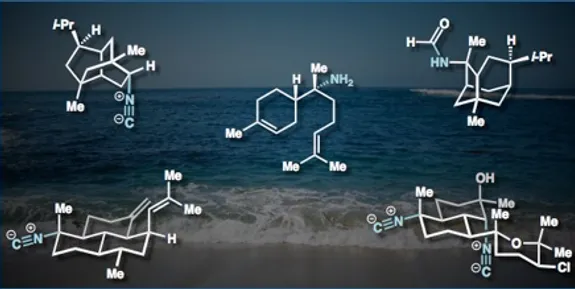
19. Jansen, D. J.; Shenvi, R. A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med. Chem., 2014, 6, 1127.
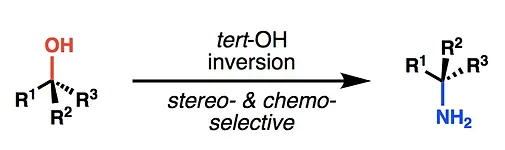
17. Pronin, S. V.; Reiher, C. A.; Shenvi, R. A. Stereoinversion of tertiary alcohols to tertiary alkyl isonitriles and amines. Nature. 2013, 501, 195-199.
- Featured by TCI Chemicals
- Highlighted by Bethany Halford in C&EN 2013, 91, 8.
- Highlighted by Stephen Davey in Nature Chemistry 2013, 5, 808.
- Highlighted by A. Räder and K. Tiefenbacher: Angew. Chem. Int. Ed. 2013, 52, 2.
- Selected for highlight in C&EN: “2013’s Notable Advances” 2013, 91, 15.
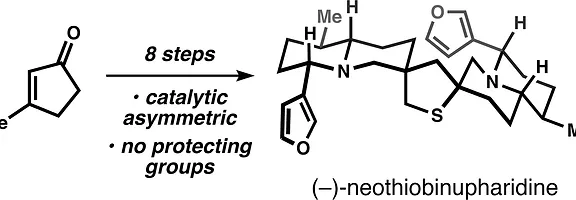
16. Jansen, D. J.; Shenvi, R. A. Synthesis of (–)-Neothiobinupharidine. J. Am. Chem. Soc. 2013, 135, 1209-1212.
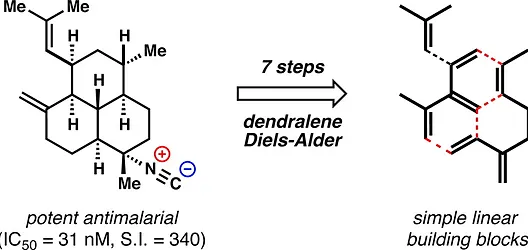
15. Pronin, S. V.; Shenvi, R. A. Synthesis of a Potent Antimalarial Amphilectene. J. Am. Chem. Soc. 2012, 134, 19604-19606.
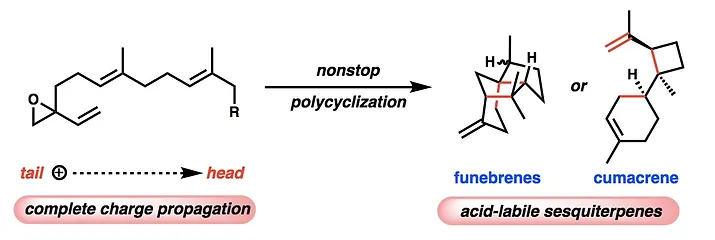
14. Pronin, S. V.; Shenvi, R. A. Synthesis of Highly Strained Terpenes by Nonstop Tail-to-Head Polycyclization. Nature Chem. 2012, 4, 915-920.
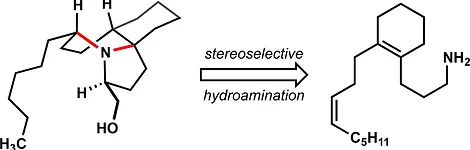
13. Pronin, S. V.; Tabor, M. G.; Jansen, D. J.; Shenvi, R. A. A Stereoselective Hydroamination Transform to Access Polysubstituted Indolizidines. J. Am. Chem. Soc. 2012, 134, 2012-2015.
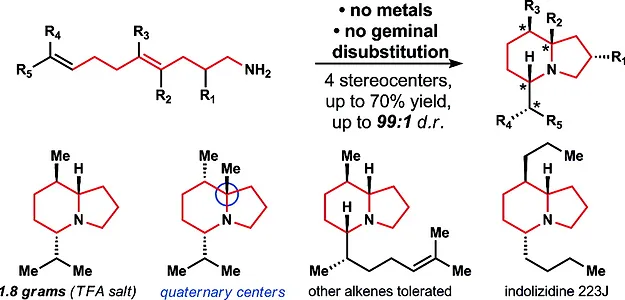
- In top ten most accessed articles of J. Am. Chem. Soc. for January 2012.
- Highlighted in Nature Chemistry, May 2012.
12. Shi, J.; Manolikakes; G. Yeh, C.-H.; Guerrero, C. A.; Shenvi, R. A.; Shigehisa, Hiroki; Baran, P. S. Scalable Synthesis of Cortistatin A and Related Structures. J. Am. Chem. Soc. 2011, 133, 8014-8027.
11. Shenvi, R. A.; Corey, E. J. Synthetic Access to Bent Polycycles by Cation-Pi Cyclization. Org. Lett. 2010, 12, 3548-3551.