Computer-aided synthesis planning (CASP)
Computer-aided synthesis planning (CASP) has become a broad term to describe the use of computation in the decision-making steps of chemical synthesis. CASP originated with retrosynthetic analysis, Corey’s problem-solving tool for multi-step synthesis, which was formulated for students and early computers alike. Retrosynthetic tools in the modern era of nascent-AI are now just beginning to make meaningful contributions to standard laboratory work.
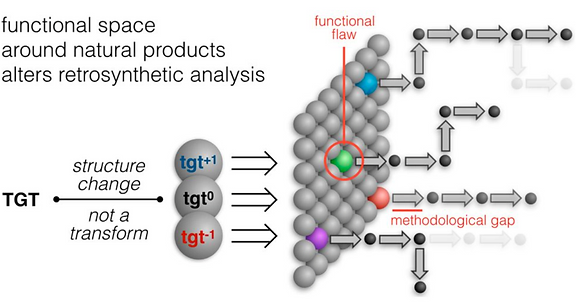
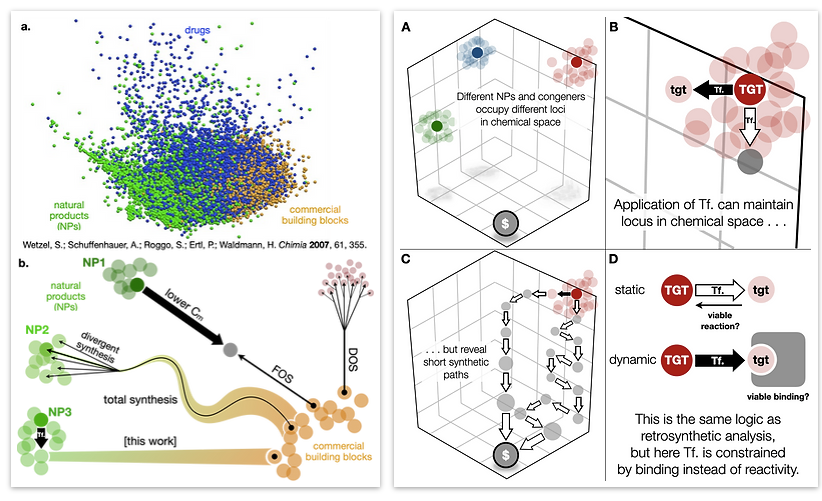
In light of these advances, our lab has proposed a merger of retrosynthetic analysis and target perturbation—a thought-experiment we call dynamic retrosynthetic analysis because the target is no longer treated as static. Instead, the strategic “moves” of transform applications can include changes to the target itself. It would be like adding, subtracting or replacing a chess piece in the middle of a game—against the rules, but very simplifying.
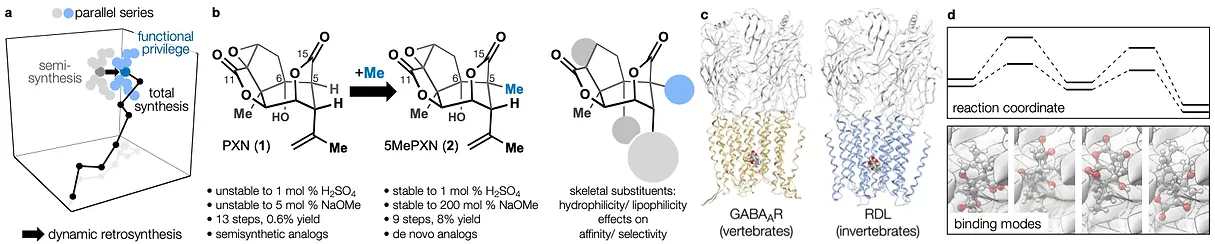
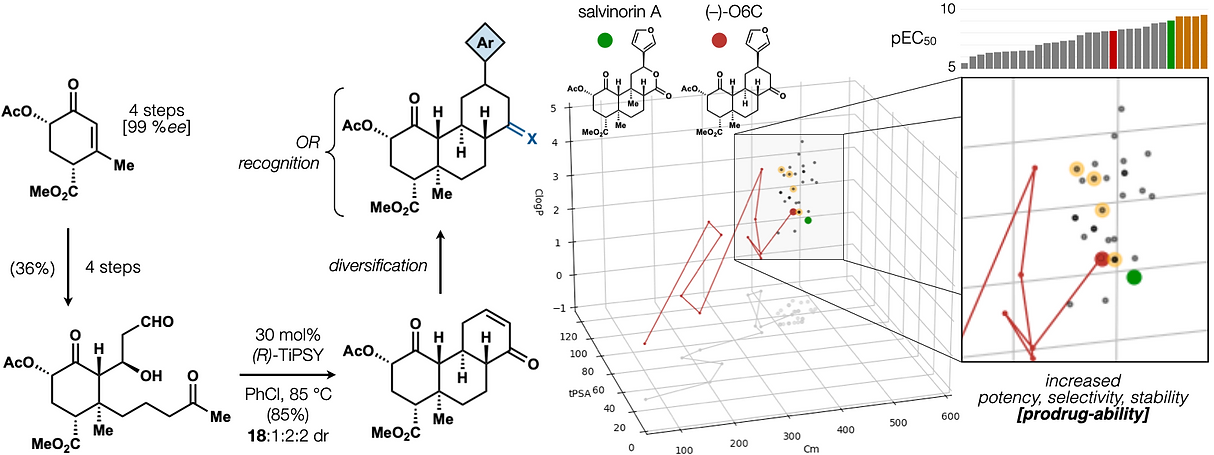
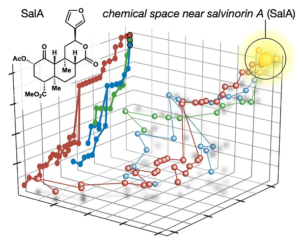
This minor difference signals a major break from the dogma of the field. Yet it retains all the benefits of traditional natural product synthesis, especially the prioritization of molecular complexity, which is intimately connected to selectivity, pharmacodynamics and pharmacokinetics. This is especially important for natural products, whose molecular parameters provide advantages for oral administration, absorption and target cell penetration. We have reduced these ideas to practice with the natural products salvinorin A (a potent KOR agonist) and picrotoxinin (a potent GABAAR antagonist).
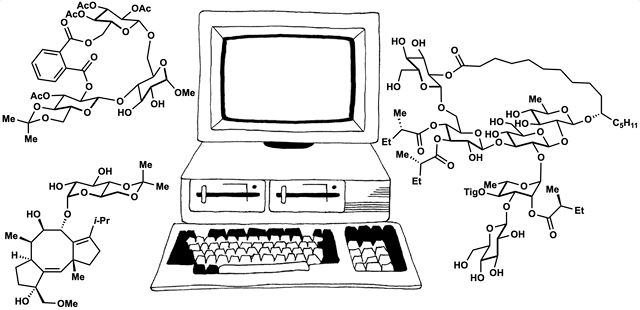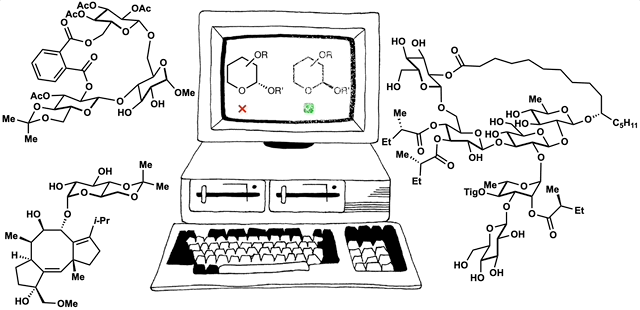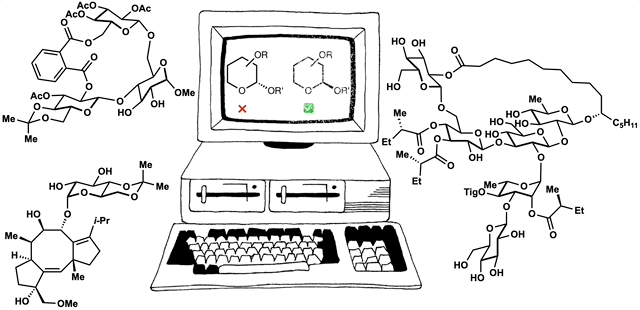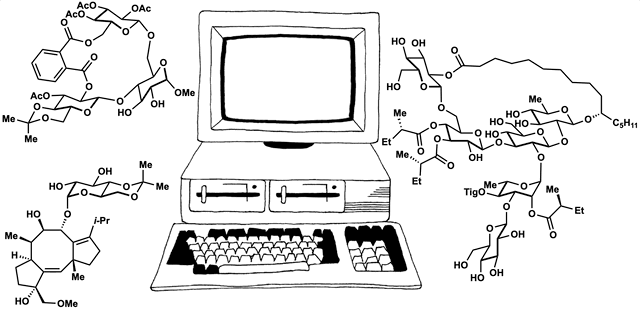
The framework of dynamic retrosynthetic analysis has also been imagined as a computational workflow called CANDOR. This workflow has many gaps that we are working to fill by introducing computational tools to our own group and to the community. These tools have included automation of Böttcher’s complexity analysis (Forli lab), in silico docking (Katrisch and Forli labs, and our own), molecular modeling, high-level DFT prediction, automated conformation searching (AutoDFT, developed Dr. Stephen Ting, a postdoc in our group) and virtual library selection using DFT and multivariate regression analysis (developed by graduate student Chunyu Li).
89. Fernandez, S. A.; Snelson, D. W.; Shenvi, R. A. “Digital Duet: Synergism between computation and the synthesis of glycosides” TCI Mail, 2025, accepted.
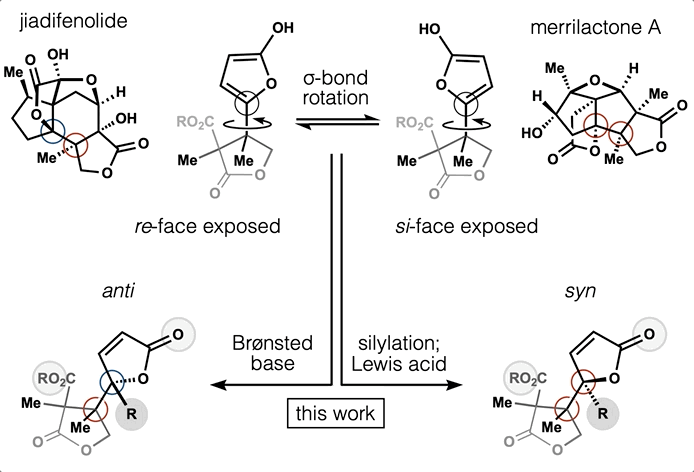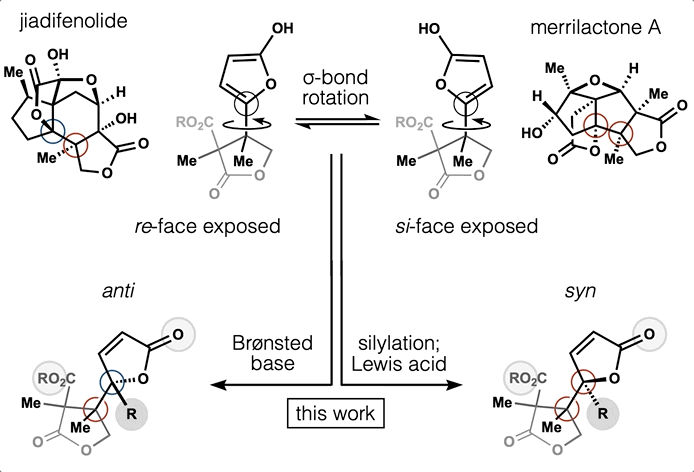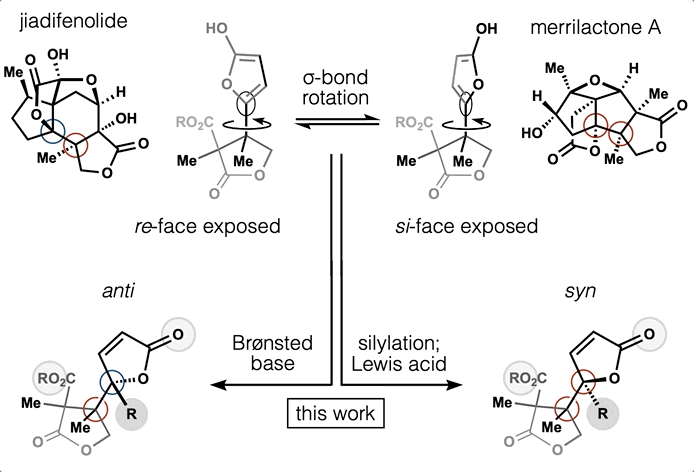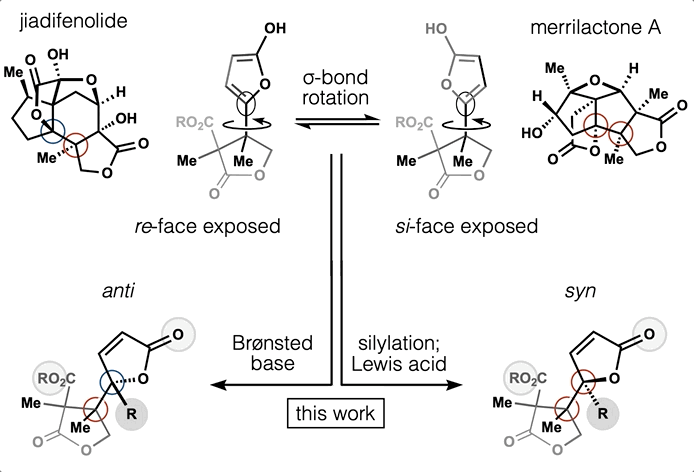
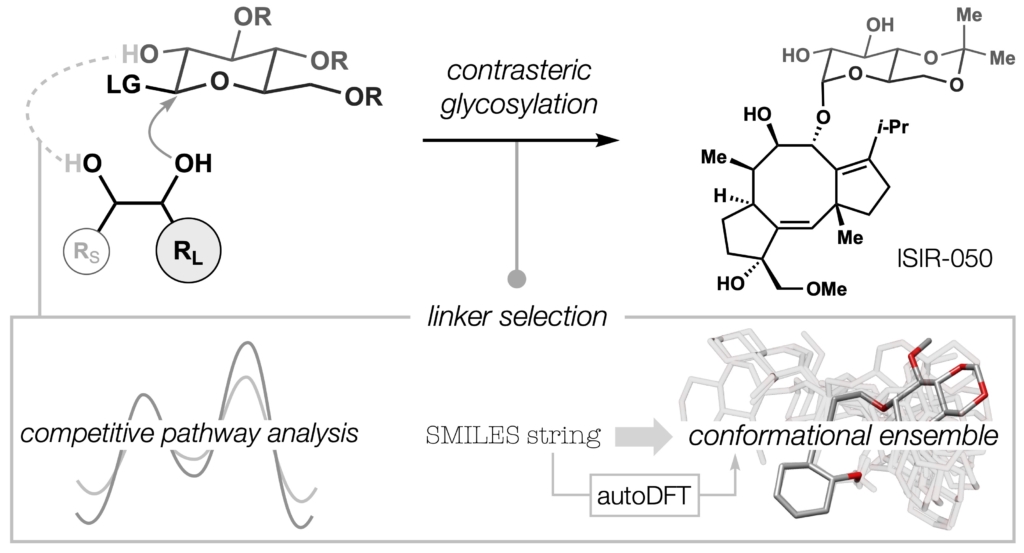
86. Snelson, D.;† Ting, S.;† Shenvi, R. A. “Contrasteric glycosylations of cotylenol and 1,2-diols by virtual linker selection” J. Am. Chem. Soc. 2025, 147, 1327–1333.
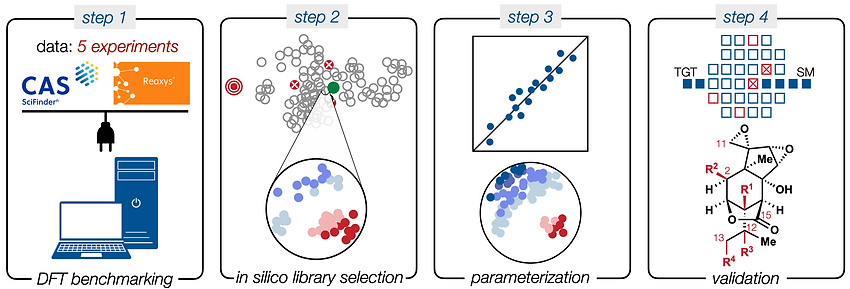
85. Li, C.; Shenvi, R. A. “Total synthesis of twenty-five picrotoxanes by virtual library selection” Nature, 2024, accepted. ChemRxiv DOI: 10.26434/chemrxiv-2024-g8j1r
79. Shenvi, R. A. “Natural product synthesis in the 21st century: beyond the mountain top” [invited Perspective] ACS Cent. Sci. 2024, 10, 519–528.

78. Tong, G.; Griffin, S.; Sader, A.; Crowell, A. B.; Beavers, K.; Watson, J.; Buchan, Z.; Chen, S.; Shenvi, R. A. C5 Methylation Confers Accessibility, Stability and Selectivity to Picrotoxinin Nature Commun. 2023, 14, 8308.
ChemRxiv DOI: 10.26434/chemrxiv-2022-0l4nt
72. Hill, S. J.§; Dao, N.§; Dang, V.; Stahl, E.; Bohn, L. Shenvi, R. A. A route to potent, selective and biased salvinorin chemical space. ACS Cent. Sci., 2023, online. §co-first authors
62. Woo, S.; Shenvi, R. A. Natural Product Synthesis through the Lens of Informatics Acc. Chem. Res. 2021, 54, 1157–1167.
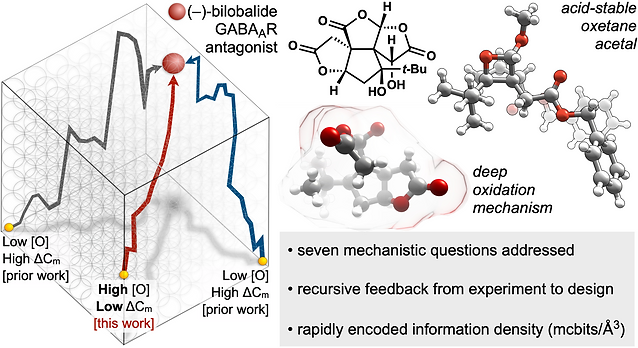
59. Demoret, R. M.; Baker, M. A.; Ohtawa, M.; Chen, S.; Lam, C.-C.; Khom, S.; Roberto, M.; Forli, S.; Houk, K.; Shenvi, R. A. Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide. J. Am. Chem. Soc. 2020, 142, 18599–18618.ChemRxiv DOI: 10.26434/ chemrxiv.12132939.v2
58. Hill, S. J.; Brion, A. U. C. M.; Shenvi, R. A. Chemical Syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020, 37, 1478–1496.

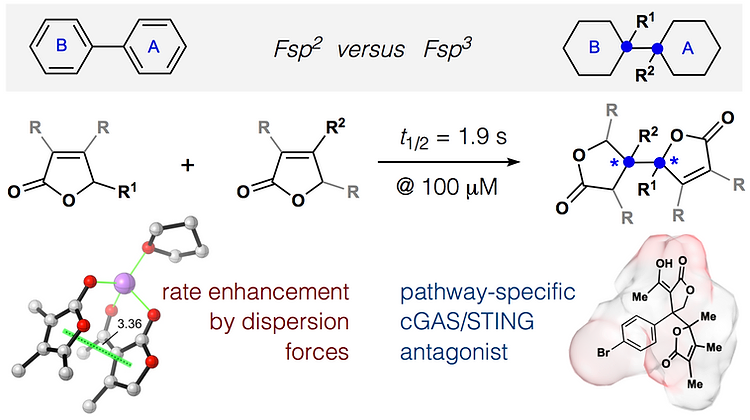
54. Huffman, B. J.; Chen, S.; Schwarz, J. L; Plata, R. E.; Chin, E.; Houk, K. N.; Lairson, L. L.; Shenvi, R. A. Electronic Complementarity Permits Hindered Butenolide Heterodimerization and Discovery of Novel cGAS/STING Pathway Antagonists, Nature Chem. 2020, 12, 310–317.
48. Huffman, B.; Shenvi, R.A. Natural Products in the ‘Marketplace’: Interfacing Synthesis and Biology J. Am. Chem. Soc. 2019, 141, 3332-3346.