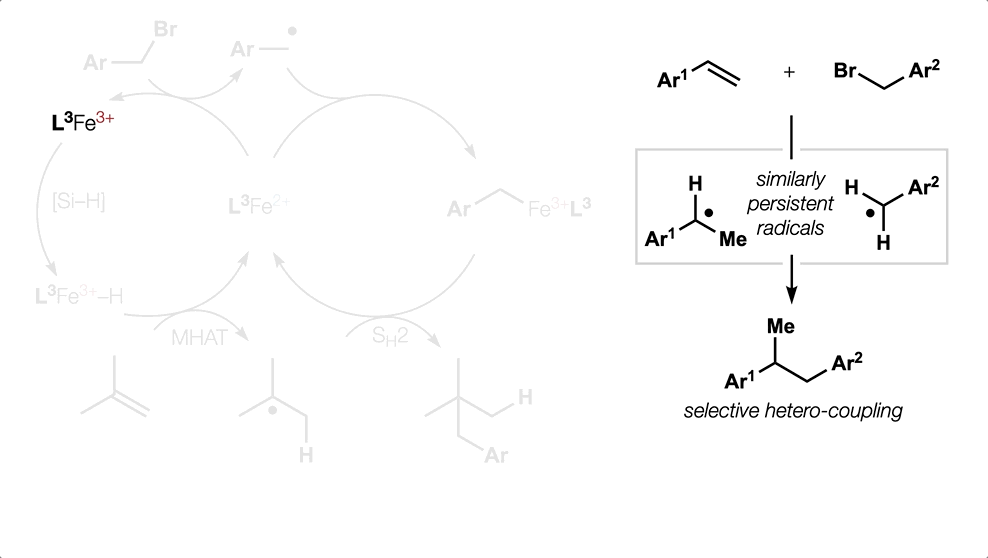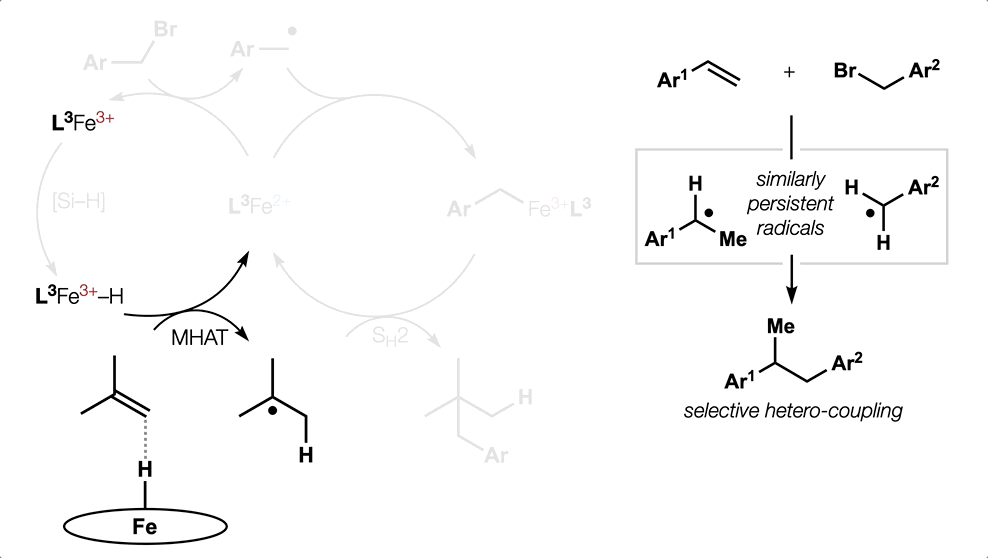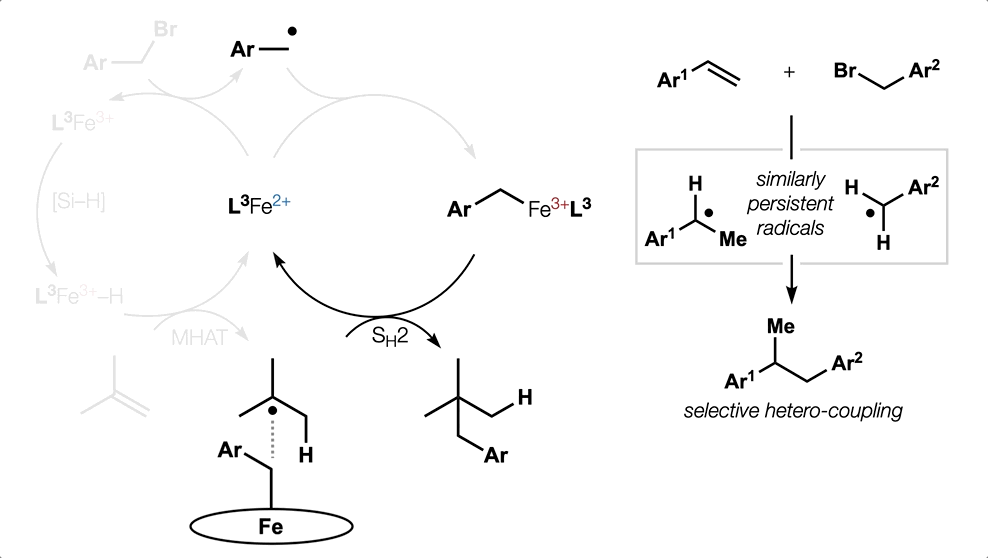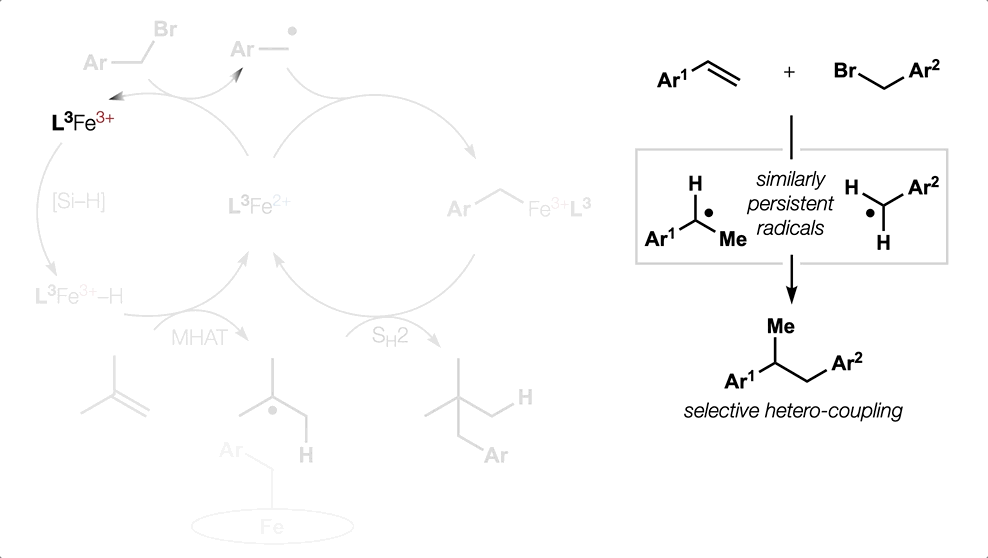
Metal Hydride (MH) Hydrogen Atom Transfer (HAT)
(or MHAT).
Classical hydrofunctionalization of alkenes requires strong Bronsted acids to form fleeting, high-energy carbocations. In contrast, non-canonical reactions of metal hydrides can hydrofunctionalize alkenes through lower-energy carbon-centered radicals. We proposed that Co, Fe and Mn-catalyzed reactions pioneered by Mukaiyama proceed through Halpern’s HAT mechanistic paradigm, uniting two literature threads that had remained separate for nearly 35 years. We have used our HAT hydrogenation and HAT isomerization to synthesize inaccessible secondary metabolites (drimane, epoxyhumulene-II, α-funebrene, 7,20-diisocyanoadociane). These reactions also have been applied in total syntheses by other groups due to their high chemoselectivity and chemofidelity (see Ref. 10 below). Our group was the first to combine MH HAT catalysis with nickel-catalyzed cross coupling, which circumvents substrate prefunctionalization, uses native functionality and expands the possibilities of each catalytic cycle. Recently, we have shown that this dual catalytic strategy for branched-selective alkene functionalization can be expanded beyond nickel to other metals. For a short course in MHAT, see these slides.
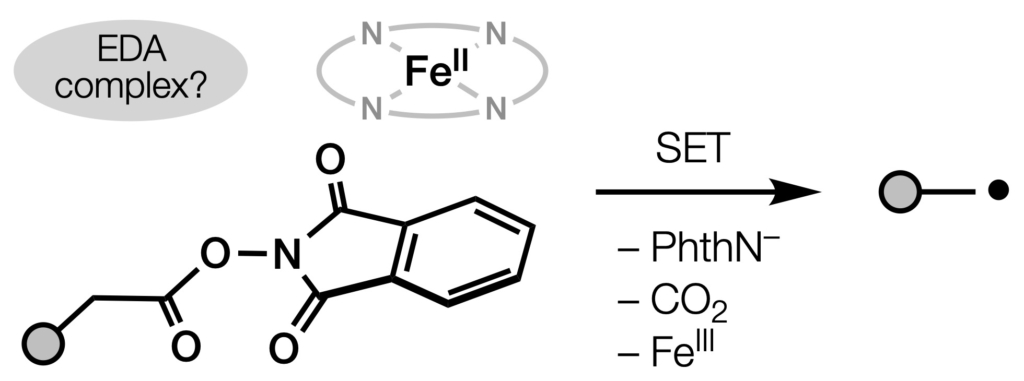
81. Dao, N.; Shenvi, R. A. “On the role of thermally activated EDA complexes in decarboxylative cross-coupling” Tetrahedron [invited, T. Maimone award issue], 2024, 166, 134204.
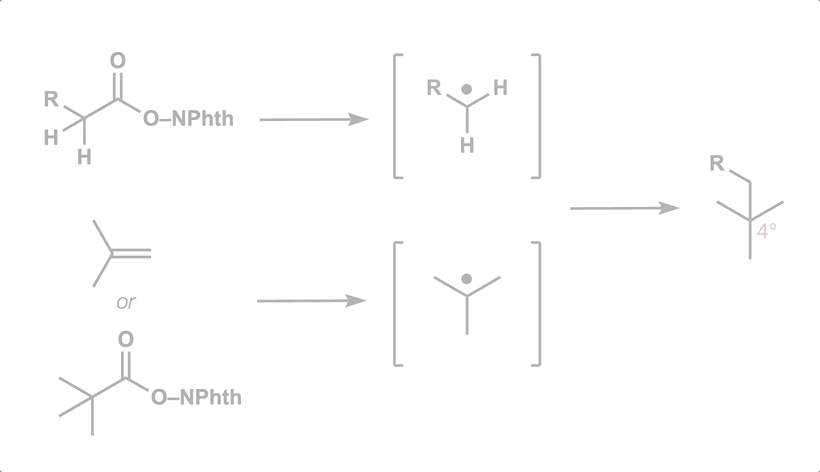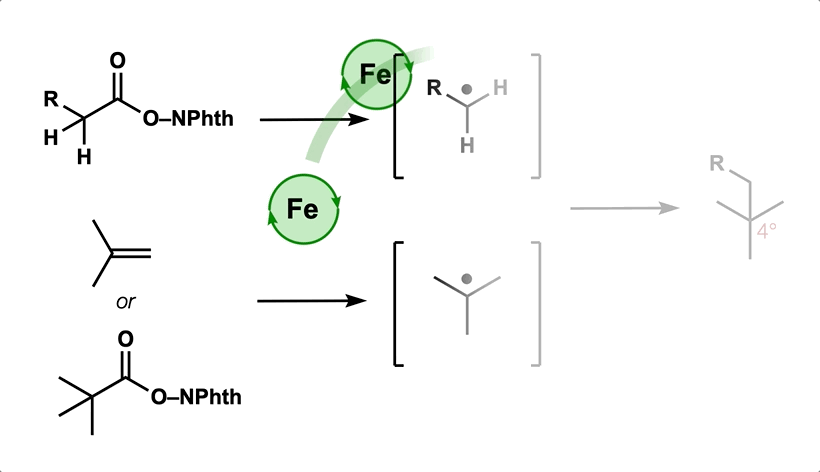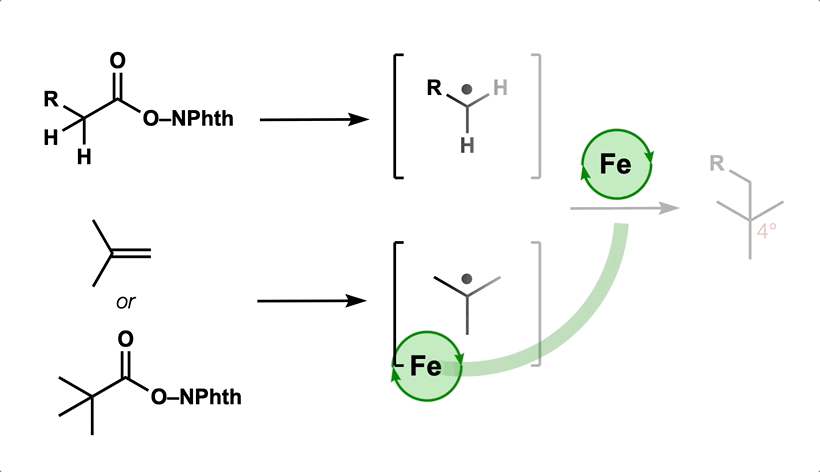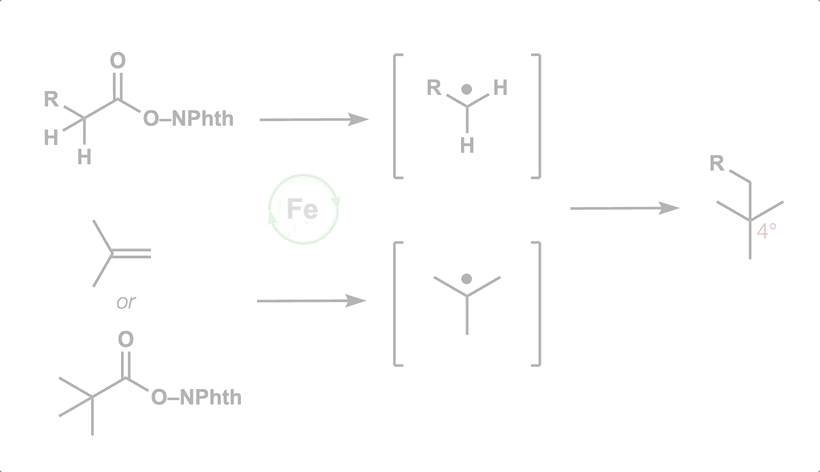
77. Gan, X.-c.;† Zhang, B.;† Dao, N.;† Bi, C.; Pokle, M.; Kan, L.; Collins, M. R.; Tyrol, C. C.; Bolduc, P. N.; Nicastri, M.; Kawamata, Y.; Baran, P. S.; Shenvi, R. A. Carbon Quaternization of Redox Active Esters and Olefins via Decarboxylative Coupling. Science 2024, 384, 113. †co-first authors
76. Kong, L.;† Gan, X.-c.;† van der Puyl-Lovett, V. A.;† Shenvi, R. A. Alkene hydrobenzylation by a single catalyst that mediates iterative outer-sphere steps. J. Am. Chem. Soc. 2024, 146, 2351.
ChemRxiv 10.26434/chemrxiv-2023-zw9c2 2023, †co-first authors.
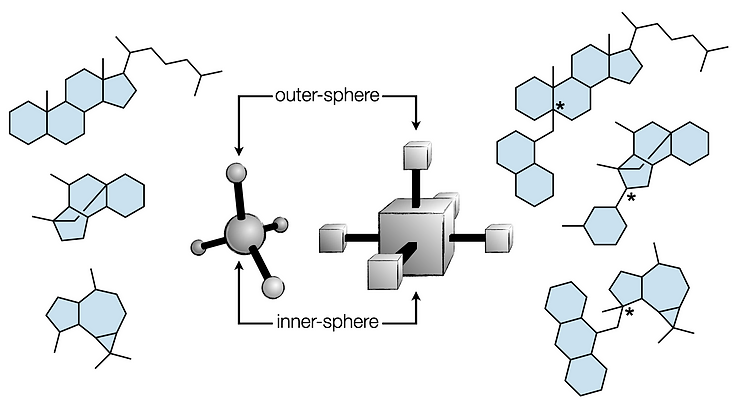
75. Kotesova, S.; Shenvi, R. A. Inner- and outer-sphere cross-couplings for high Fsp3 fragments in natural product space. Acc. Chem. Res. 2023, 56, 3089.
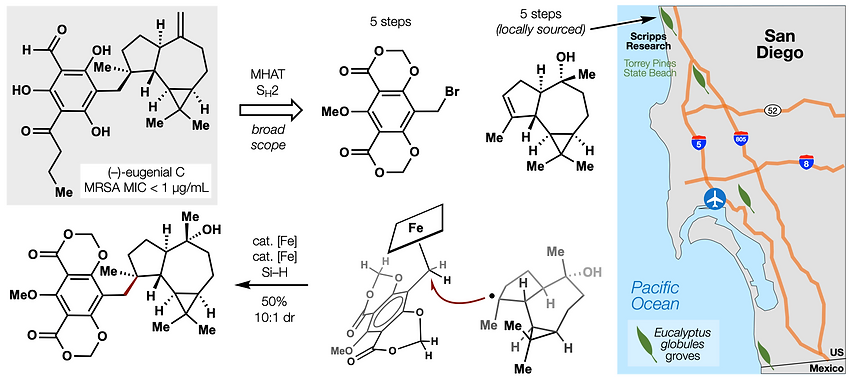
73. Gan, X.-c.;§ Kotesova, S.;§ Castanedo, A.; Green, S. A.; Møller, S. L. B.; Shenvi, R. A. Iron-catalyzed hydrobenzylation: stereoselective synthesis of (–)-eugenial C. J. Am. Chem. Soc. 2023, 145, 15714. §co-first authors
70. van der Puyl, V. A.; Shenvi, R. A. Manganese, iron and cobalt-catalyzed radical olefin hydrofunctionalization Science of Synthesis, 2.14: Base Metal Catalysis, 2023, Ed. N. Yoshikai.
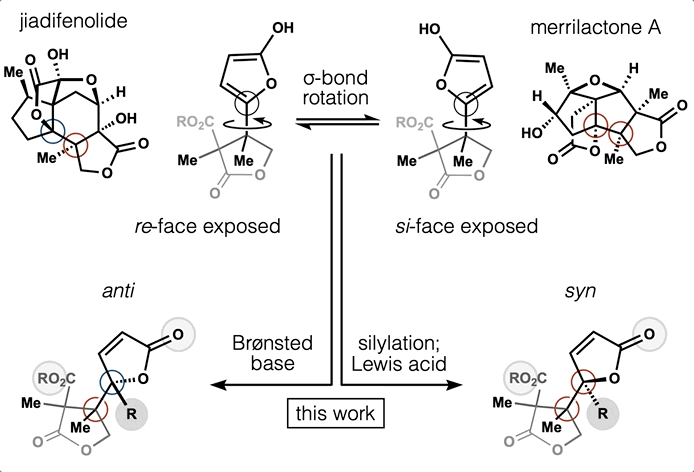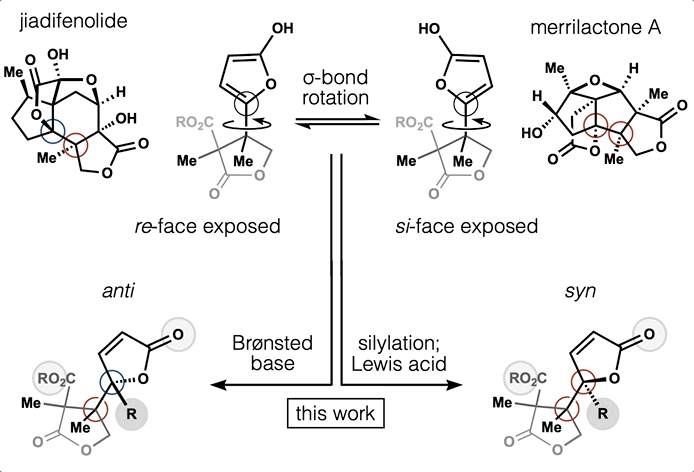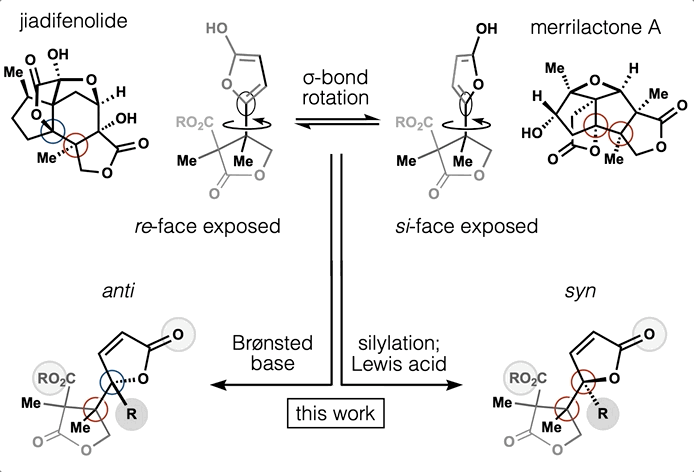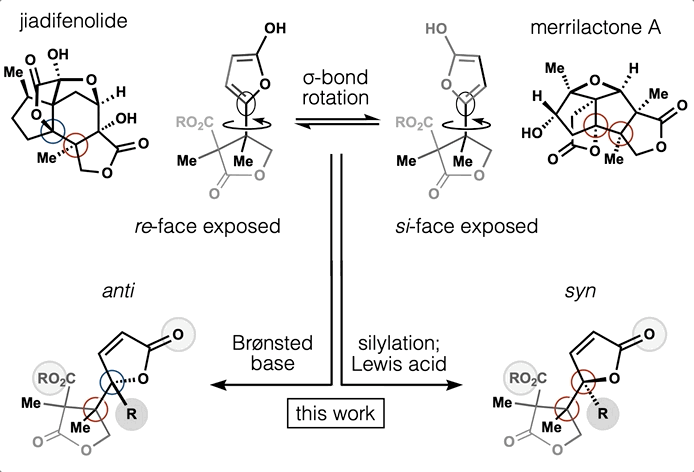
65. Huffman, B. J.; Chu, T.; Hanaki, Y.; Wong, J. J.; Chen, S.; Houk, K. N.; Shenvi, R. A. Stereodivergent attached ring synthesis via non-covalent interactions: a short formal synthesis of merrilactone A Angew. Chem. Int. Ed. 2022, 61, e202114514.
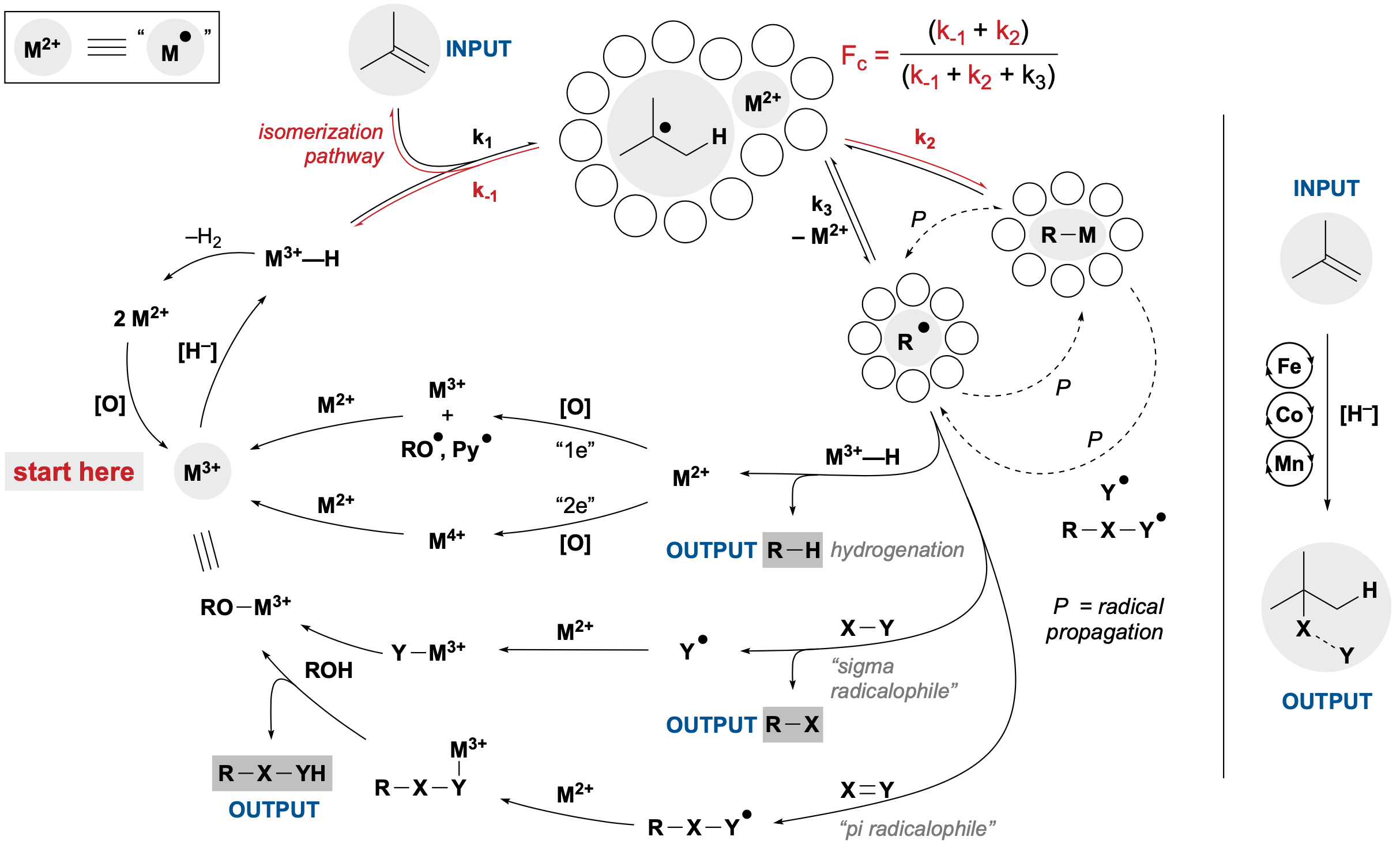
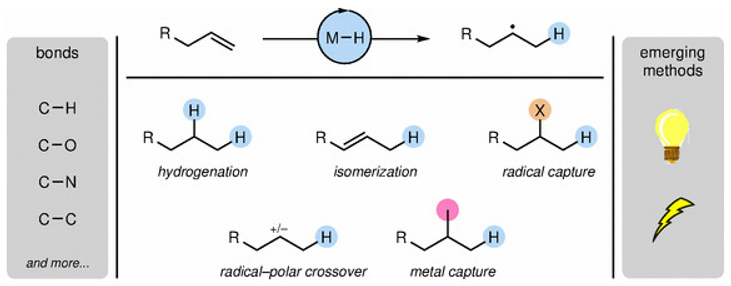
60. Shevick, S. L.; Wilson, C. V.; Kotesova, S.; Kim, D.; Holland, P. L.; Shenvi, R. A. Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals. Chem. Sci. 2020, 12401–12422.
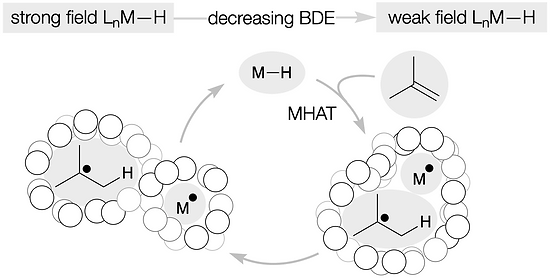
56. Matos, J. L. M.; Green, S. A.; Chun, Y; Dang, V. Q.; Dushin, R. G.; Richardson, P.; Chen, J. S.; Piotrowski, D. W.; Paegel, B. M.; Shenvi, R. A. Cycloisomerization of olefins in water, Angew. Chem. Int. Ed. 2020, 59, 12998–13003.
ChemRxiv: 10.26434/chemrxiv.11977917.v1
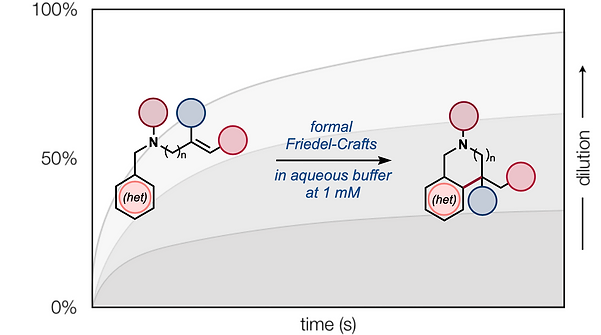
53. Shevick, S. L.; Baker, M. A.; Shenvi, R. A. Alkene Hydroarylation by Co/Ni Dual Catalysis, Cell: Trends in Chemistry, 2019, 1, 540–541.
52. Matos, J. L. M.; Green, S. A.; Shenvi, R. A. Chapter 7. Markovnikov Functionalization by Hydrogen Atom Transfer, Organic Reactions, 2019, 100, 383–470.
50. Green, S. A.; Huffman, T. R.; McCourt, R. O.; van der Puyl, V.; Shenvi, R. A. Hydroalkylation of Olefins to form Quaternary Carbons J. Am. Chem. Soc. 2019, 141, 7709-7714.
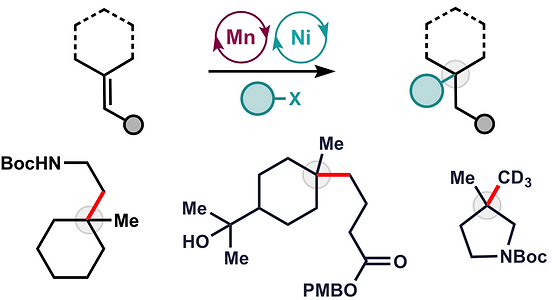
46. Matos, J.L.M.; Vásquez-Céspedes, S.; Gu, J.; Oguma, T.; Shenvi, R.A. Branch-Selective Addition of Unactivated Olefins into Imines and Aldehydes J. Am. Chem. Soc. 2018, 140, 16976–16981.
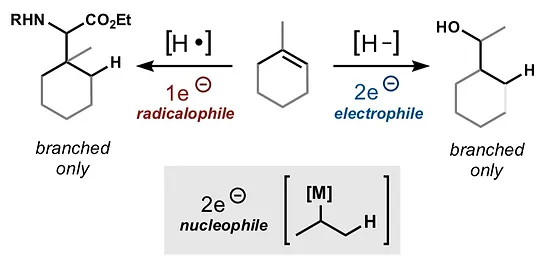
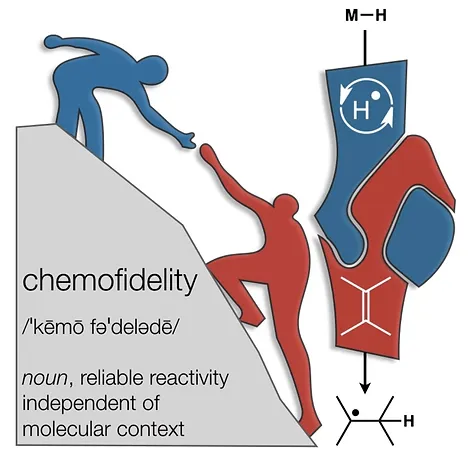
45. Green, S.A.; Crossley, S.W.M.; Matos, J.L.M.; Vásquez-Céspedes, S.; Shevick, S. L.; Shenvi, R. A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer Acc. Chem. Res., 2018, 51, 2628–2640.
44. Shevick, S. L.; Obradors, C.; Shenvi, R. A. Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation J. Am. Chem. Soc. 2018,140, 12056–12068.
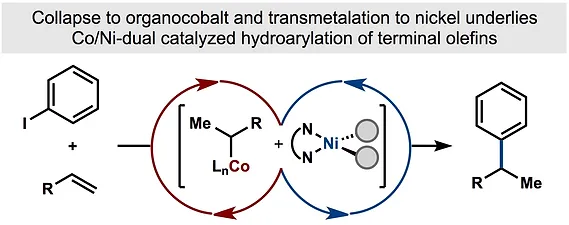
43. Green, S. A.; Vásquez-Céspedes, S.; Shenvi, R. A. Iron-Nickel Dual-Catalysis: A New Engine for Olefin Functionalization. J. Am. Chem. Soc. 2018, 140, 11317–11324
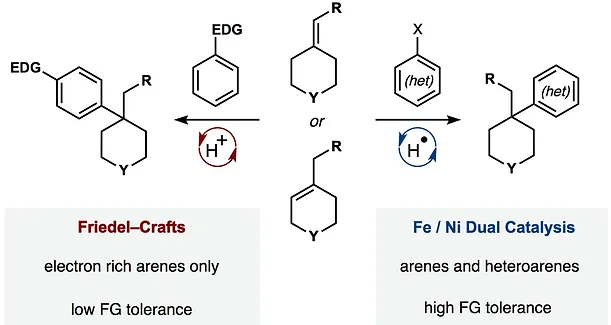
35. Green, S. A.; Matos, J. L. M.; Yagi, A.; Shenvi, R. A. Branch-Selective Hydroarylation: Iodoarene-Olefin Cross Coupling. J. Am. Chem. Soc. 2016, 138, 12779-12782.
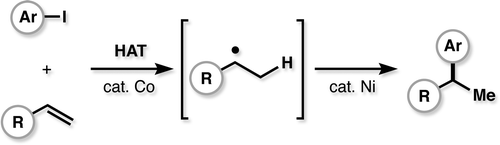
33. Crossley, S. W. M.; Martinez, R. M.; Obradors, C.; Shenvi, R. A. Mn, Fe, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912-9000.
30. Crossley, S. W. M.; Martinez, R. M.; Zuluaga, S. G.; Shenvi R. A. Synthesis of the Privileged 8-Arylmenthol Class by Radical Arylation of Isopulegol. Org. Lett. 2016, 18, 2620-2623.
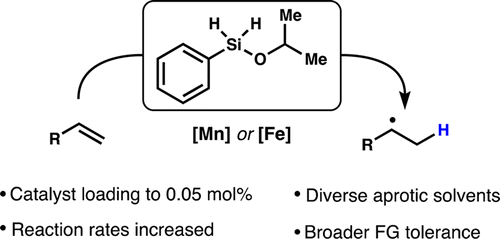
29. Obradors, C. L.; Martinez, R.; Shenvi, R. A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016, 138, 4962-4971.
27. Shenvi, R. A. Reinventing Radical Reactions. SynLett Cluster (Ed. T. Rovis and R. A. Shenvi). 2016, 27, 678-679.
20. Crossley, S. W. M.; Barabé, F.; Shenvi, R. A. Simple, Chemoselective, Catalytic Olefin Isomerization. J. Am. Chem. Soc. 2014, 136, 16788.
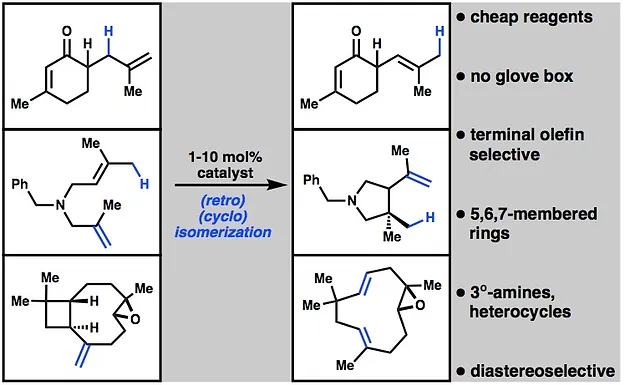
Isomerization pre-catalyst now available from Sigma-Aldrich
18. Iwasaki, K.; Wan, K. K.; Oppedisano, A.; Crossley, S. W. M.; Shenvi R. A. Simple, Chemoselective Hydrogenation with Thermodynamic Stereocontrol. J. Am. Chem. Soc. 2014, 136, 1300-1303.
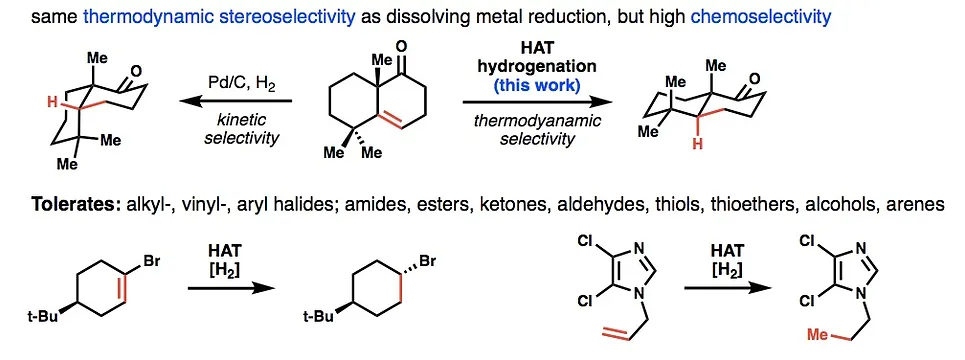
- Hydrogenation catalyst available from Sigma-Aldrich
- Highlighted by Carmen Drahl in C&EN 2014, 92, 9.