Your brain is chemical…
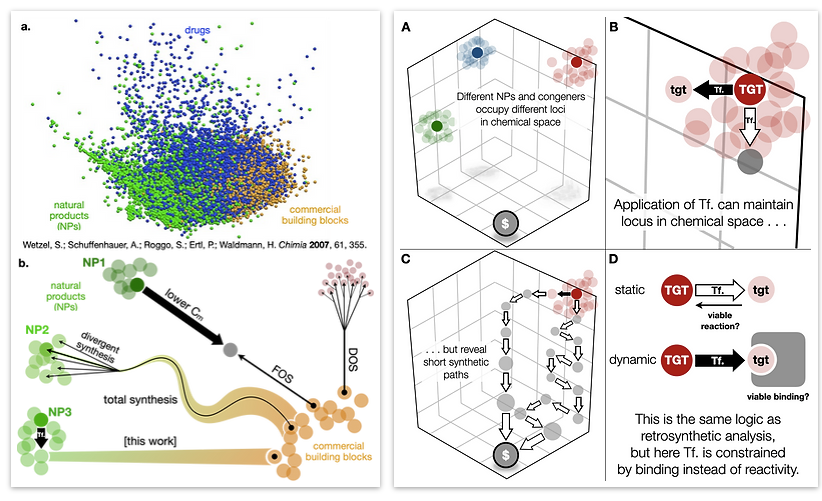
Small molecules mediate its communication, maintenance, function and dysfunction. Our lab has published methods to synthesize important classes of CNS-active metabolites including potent nAChR inhibitors and Illicium terpenes, sometimes called ‘neurotrophic terpenes.’ We proposed that these latter metabolites enhance neurite outgrowth through binding to the CysLoop family of neurotransmitter-gated ion channels–probably GABAa receptors. Our chemistry allows us to match the combinatorial nature of these receptors with a combinatorial assembly of terpenes. More recently, we described a computational workflow called CANDOR (Computed Affinity / Dynamically Ordered Retrosynthesis) to rapidly synthesize a stabilized salvinorin A analog that potently and selectively agonizes the kappa opioid receptor (KOR), a target for next generation analgesics and psychotropic therapeutics. This synthesis is being used to generate important KOR agonists, but we anticipate the CANDOR workflow will accelerate the deployment of many other natural product scaffolds in medicinal chemistry campaigns. For example, we recently showed that dynamic retrosynthetic methylation stabilizes the GABAaR antagonist picrotoxinin and increases its structural complexity, but also opens a dramatically shortened, eight-step route.
90. Freeman, S. M.; Landwehr, E. M.; Rojas, J. R.; Tanaka, R.; Bailey, J. B.; Gembicky, M.; Shenvi, R. A.* “Decagram-scale synthesis of GB13, a Galbulimima alkaloid precursor” 2025, ChemRxiv doi: 10.26434/chemrxiv-2025-490r9

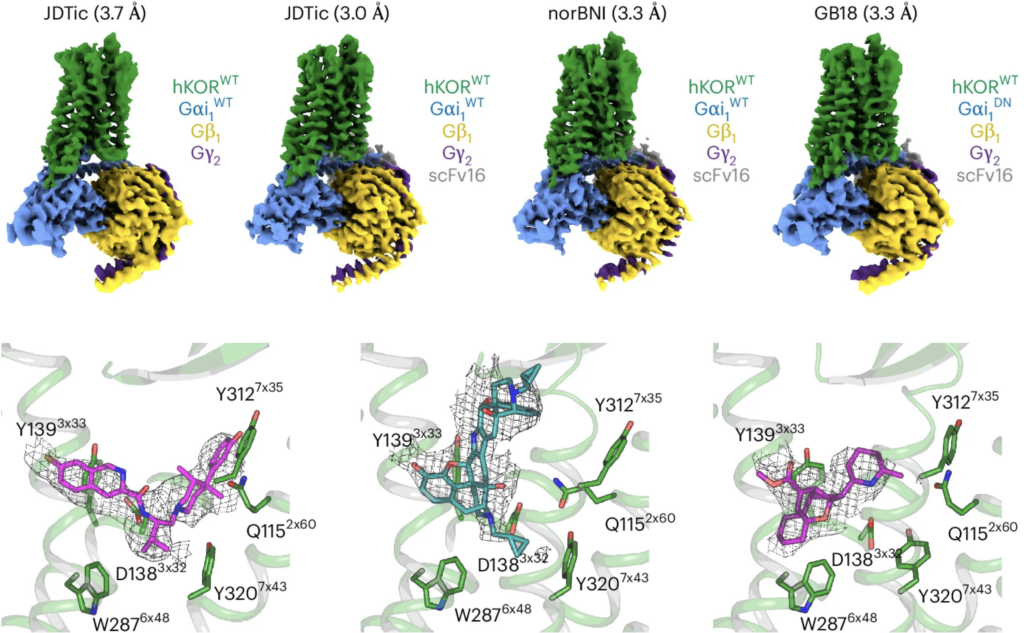
84. Motiwala, Z.; Tyson, A. S.; Styrpejko, D.; Han, G. W.; Khan, S.; Ramos-Gonzalez, N.; Woo, S.; Villers, K.; Landaker, D.; Shenvi, R. A.; Majumdar, S.; Gati, C. “Structural basis of inverse agonism via inactive kappa opioid receptor:G protein complexes” Nature Chem. Bio. 2025, 21, 1046–1057.
82. Clay, K.; Shenvi, R. A. “The original caretakers of salvinorin A” Nature Chem.2024, 16, 1735–1736.
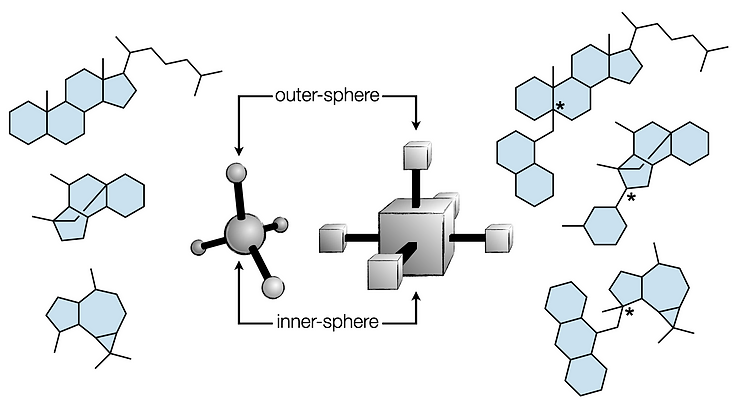
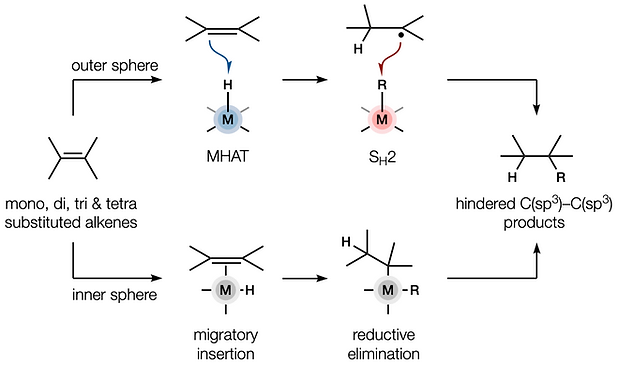
80. Dao, N.; Gan, X.-c.; Shenvi, R. A. “Metal-Hydride C–C Cross-Coupling of Alkenes Through a Double Outer-Sphere Mechanism” [invited] J. Org. Chem. 2024, 89, 16106–16113.
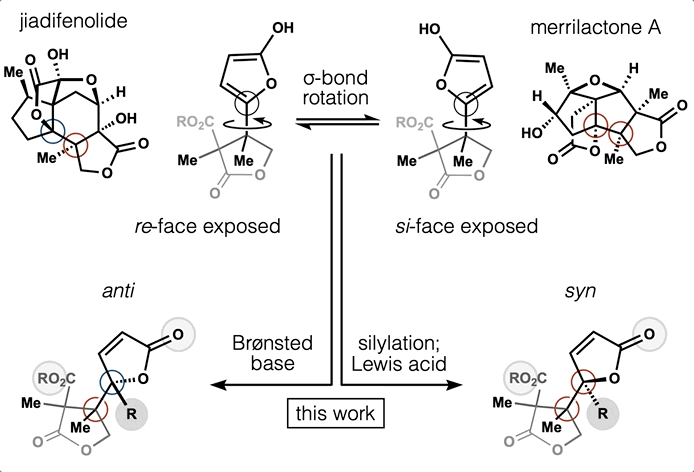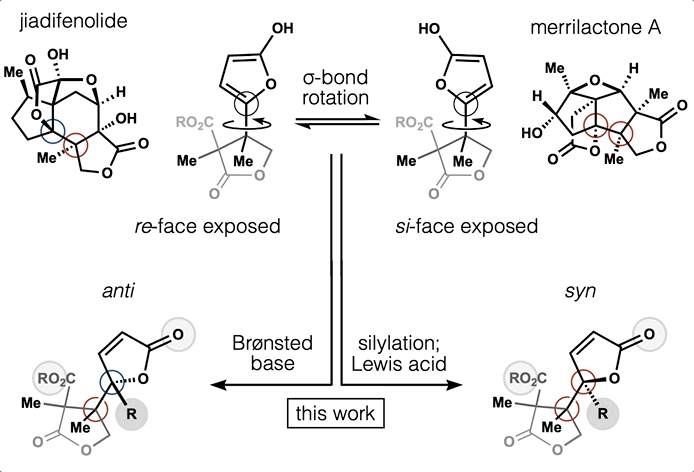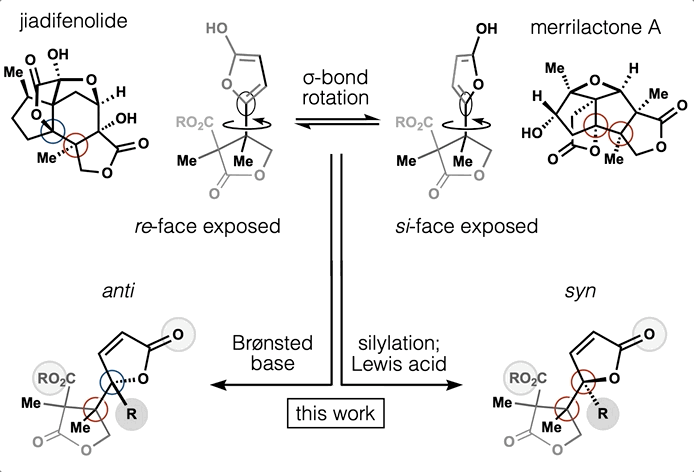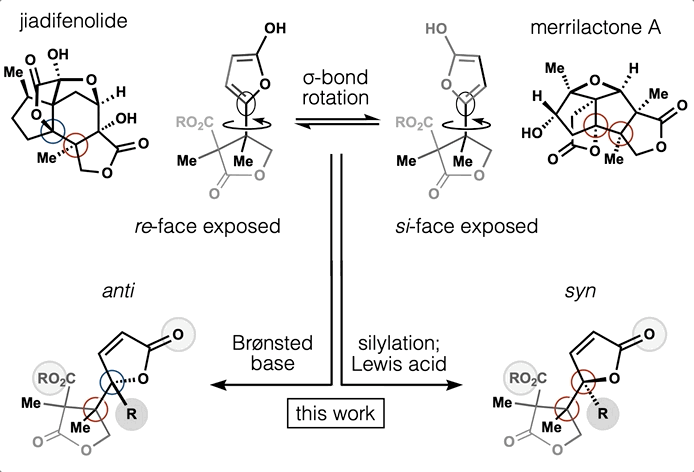
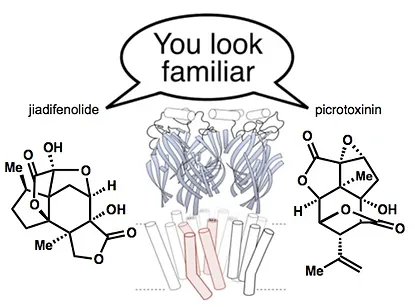
78. Tong, G.; Griffin, S.; Sader, A.; Crowell, A. B.; Beavers, K.; Watson, J.; Buchan, Z.; Chen, S.; Shenvi, R. A. C5 Methylation Confers Accessibility, Stability and Selectivity to Picrotoxinin Nature Commun. 2023, 14, 8308.
ChemRxiv DOI: 10.26434/chemrxiv-2022-0l4nt
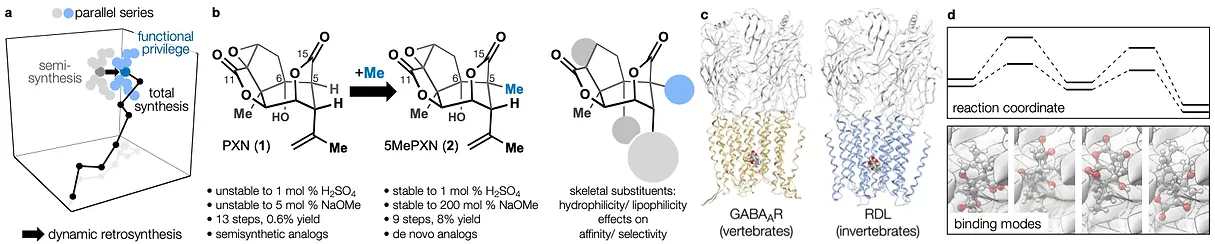
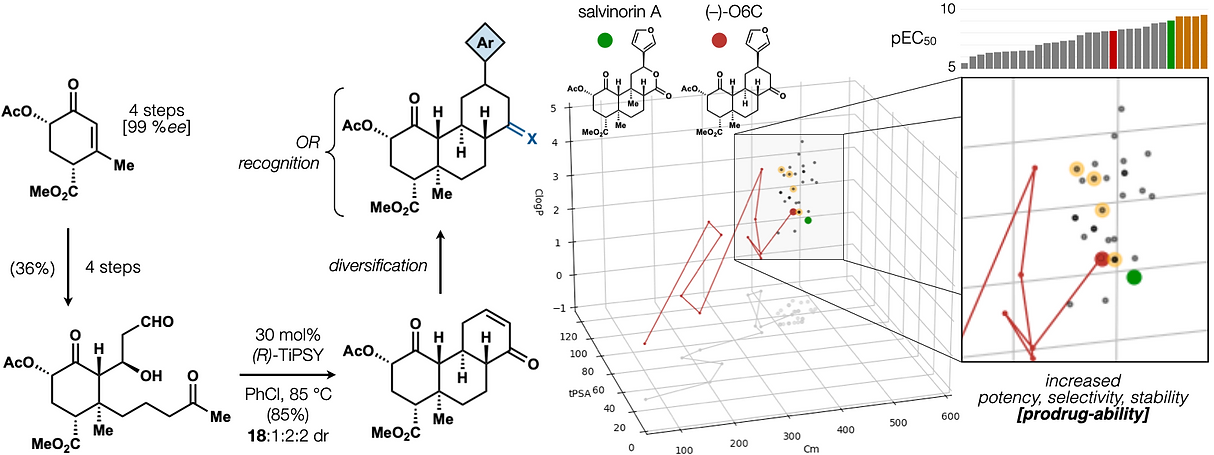
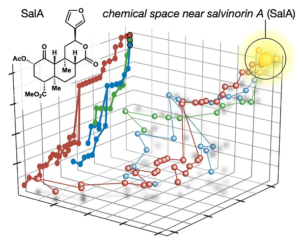
72. Hill, S. J.§; Dao, N.§; Dang, V.; Stahl, E.; Bohn, L. Shenvi, R. A. A route to potent, selective and biased salvinorin chemical space. ACS Cent. Sci., 2023, online. §co-first authors
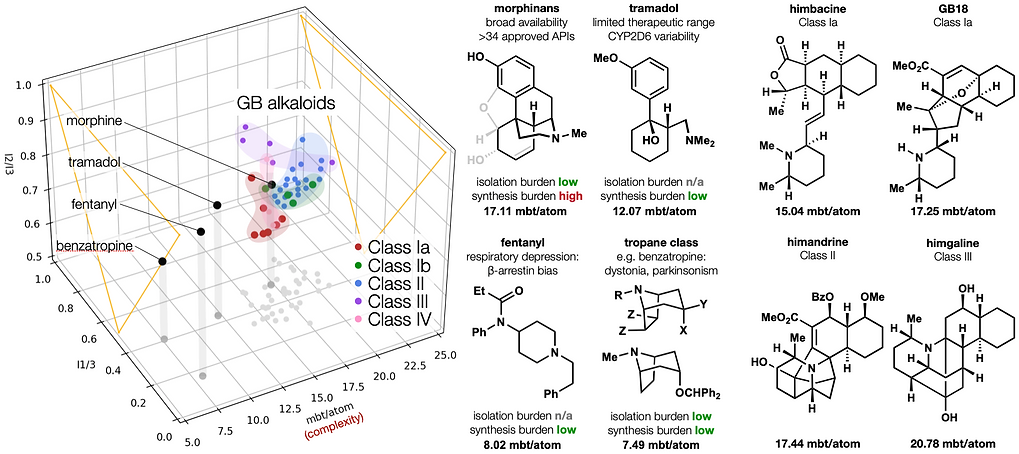
69. Woo, S.; Landwehr, E.; Shenvi, R. A. Synthesis of psychotropic alkaloids from Galbulimima Tetrahedron 2022, 126, 133064. [In celebration of the 65th anniversary of Tetrahedron Publications]
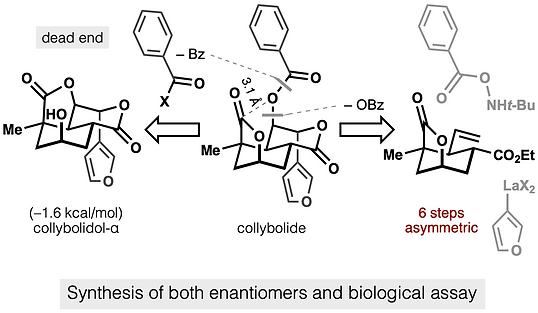
68. Shevick, S. L.; Freeman, S.; Tong, G.; Russo, R. J.; Bohn, L. M.; Shenvi, R. A. Asymmetric syntheses of (+)- and (–)-collybolide enable re-evaluation of kappa-opioid receptor agonism ACS Cent. Sci., 2022, 8, 948–954. DOI: https://doi.org/10.1021/acscentsci.2c00442.
ChemRxiv DOI: 10.26434/chemrxiv-2021-rtxqf
67. Woo, S.; Shenvi, R. A. Synthesis and target annotation of GB18. Nature, 2022, DOI: 10.1038/s41586-022-04840-9.
ChemRxiv DOI: 10.26434/chemrxiv-2022-cs8v5
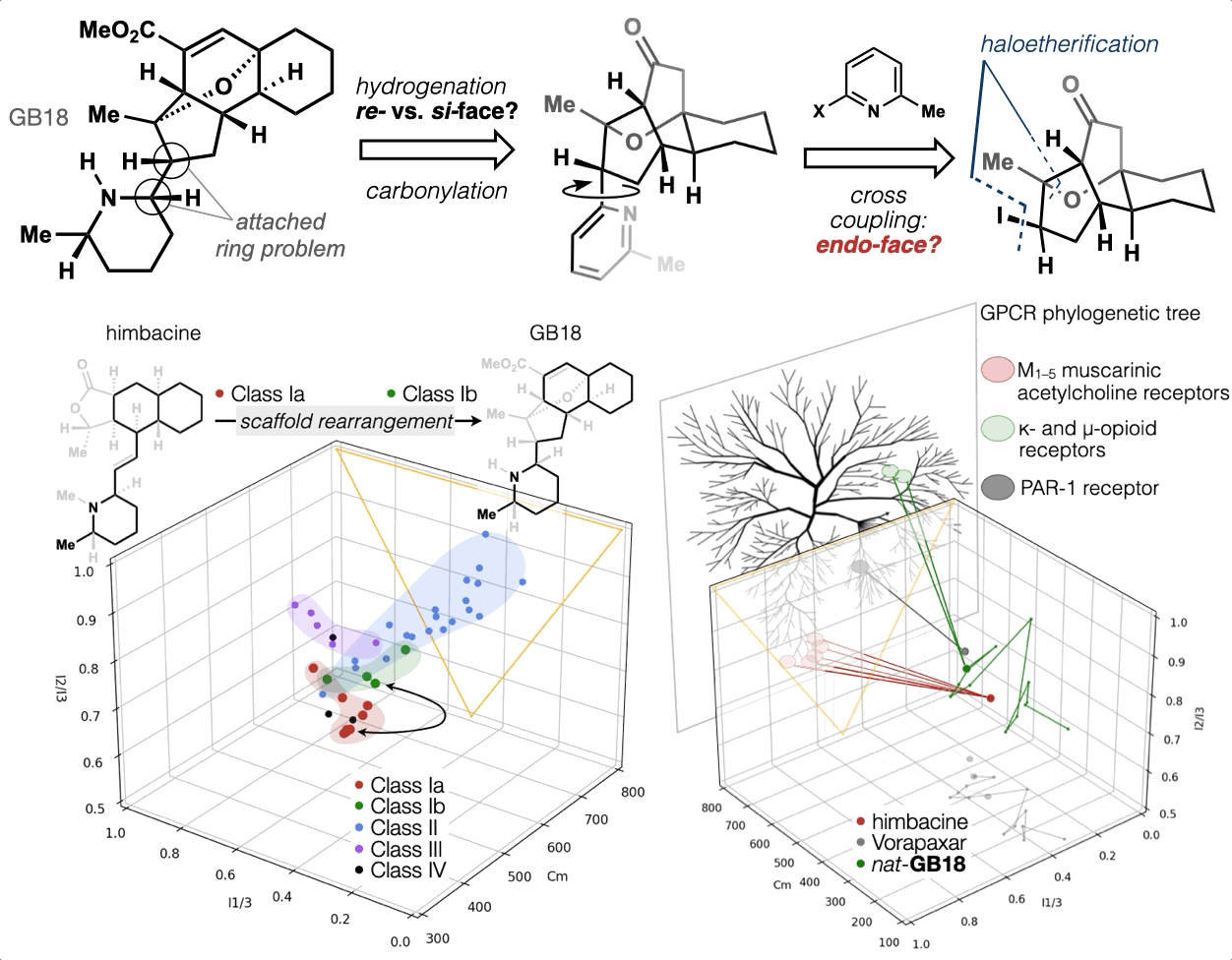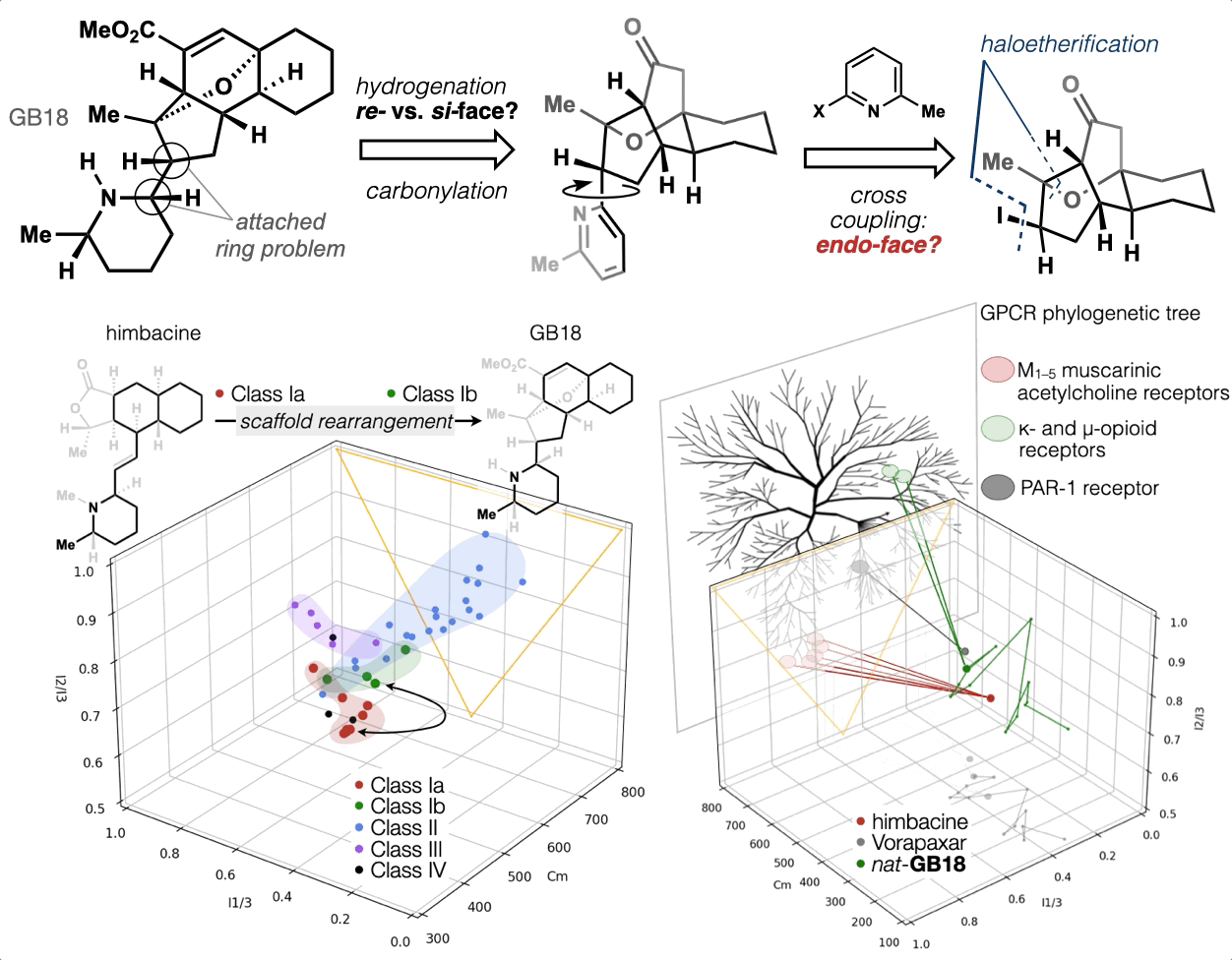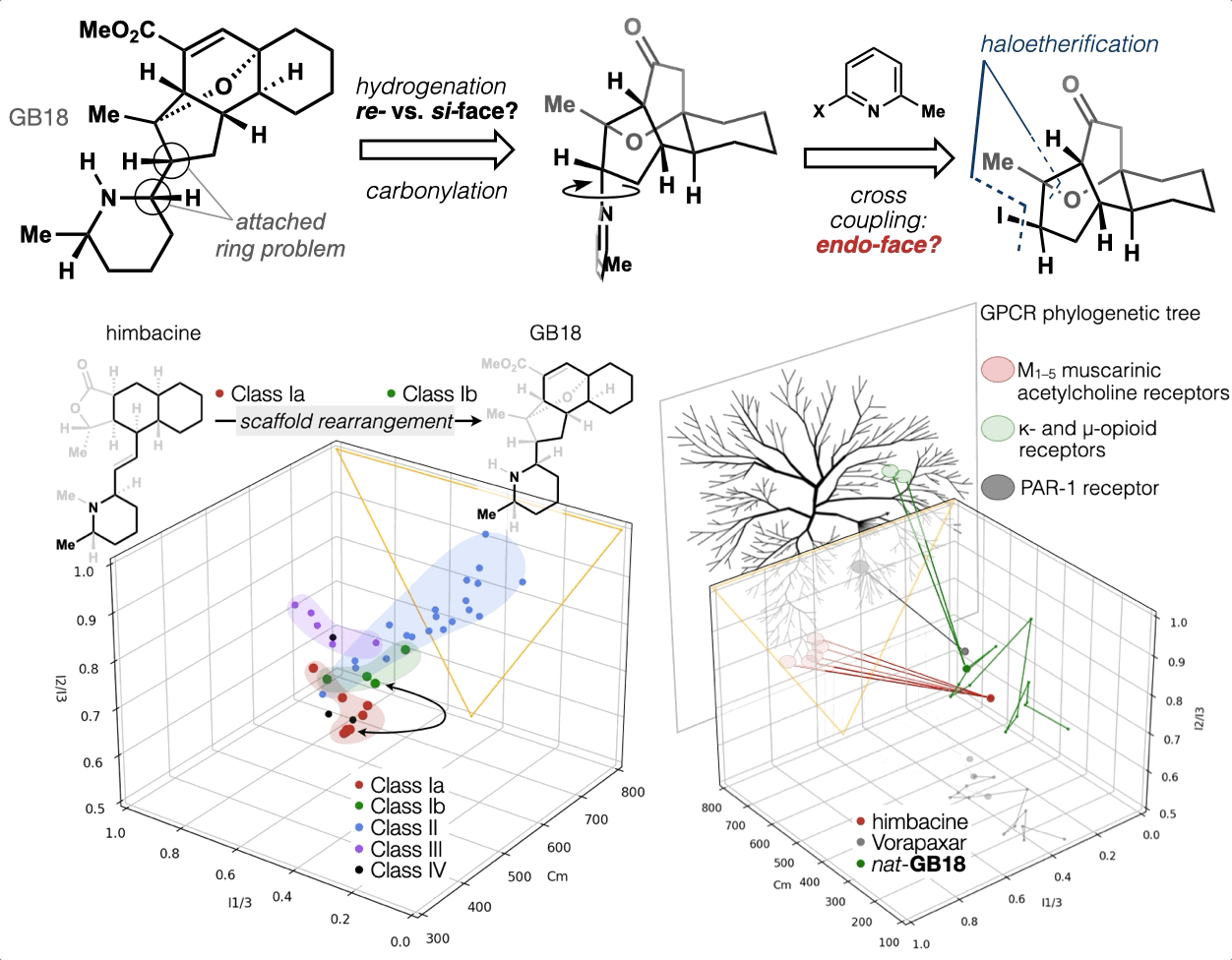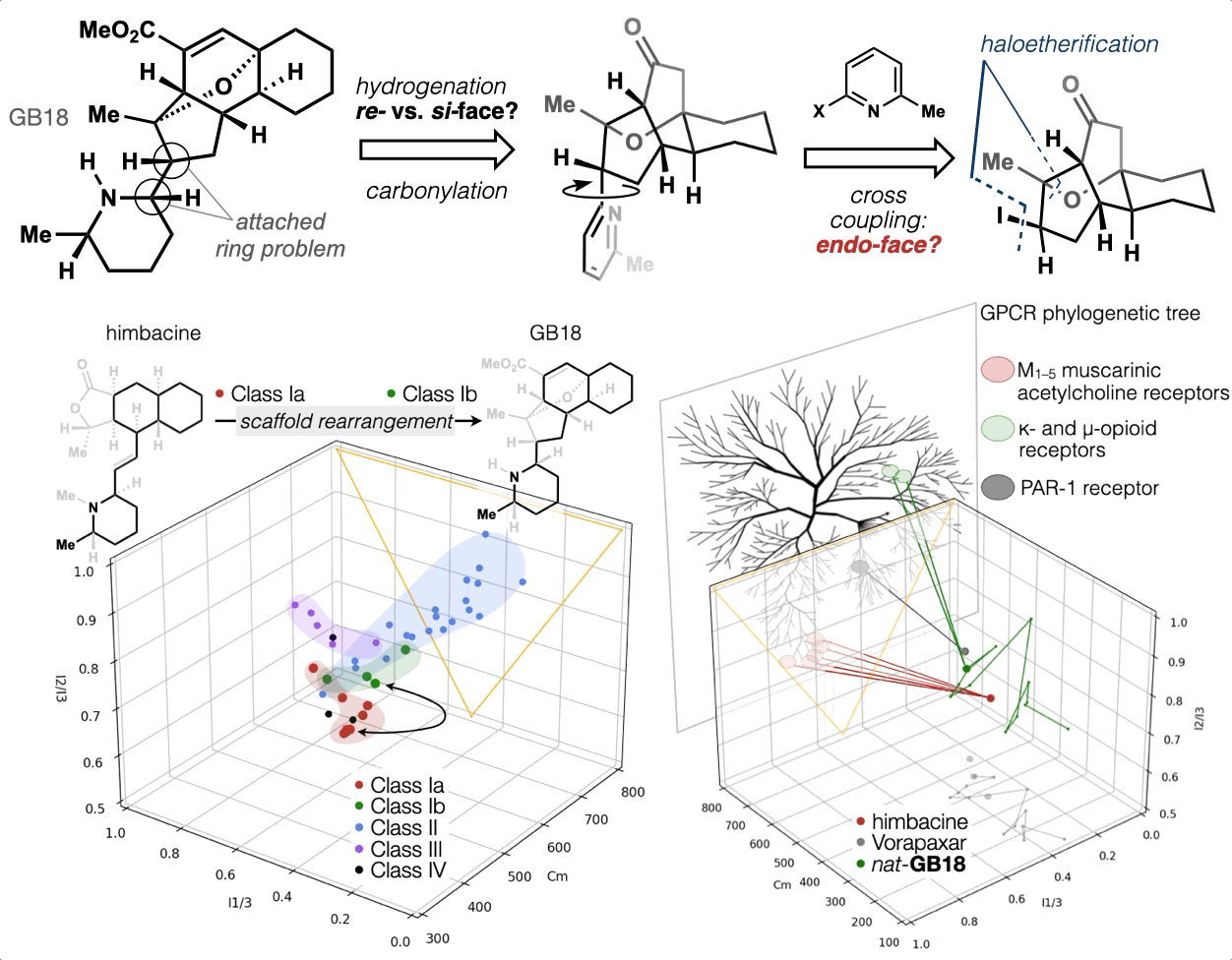
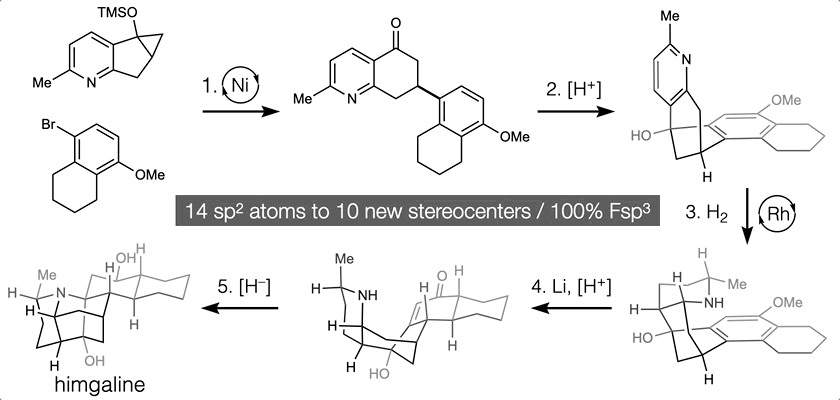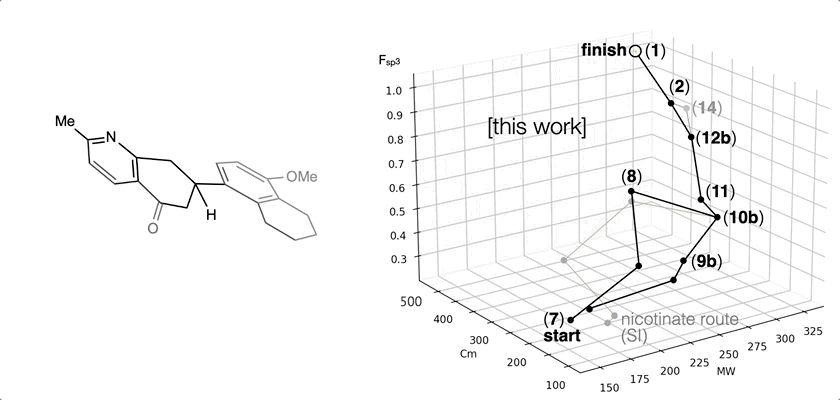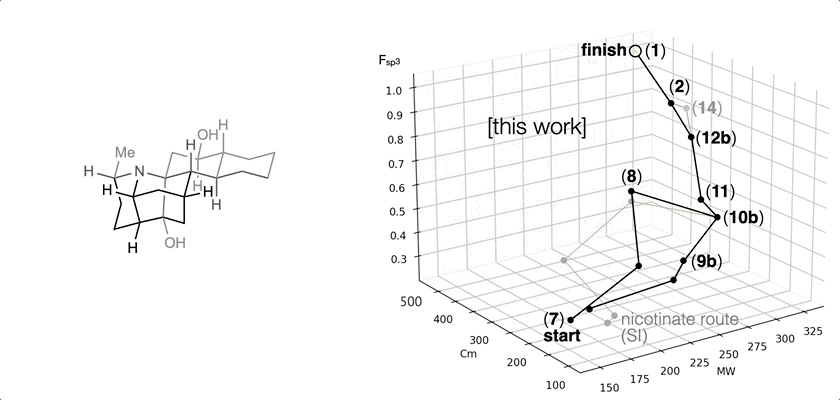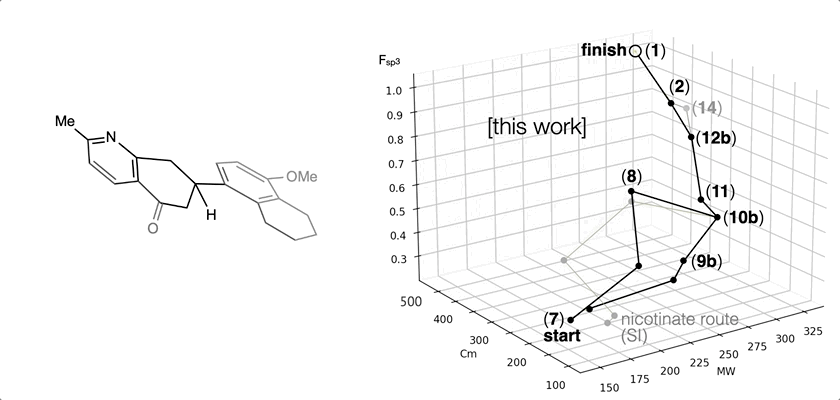
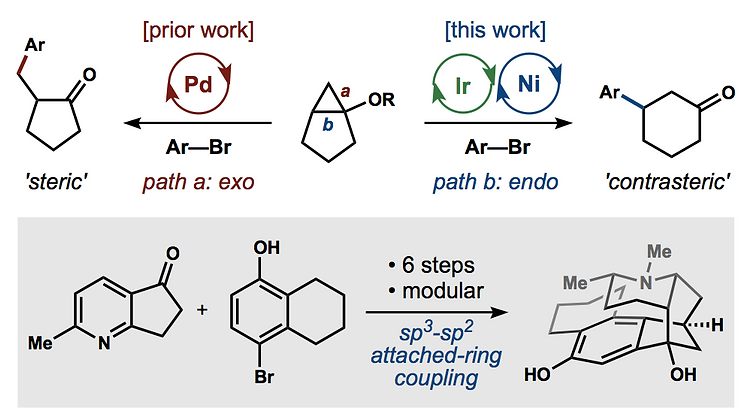
66. Landwehr, E. M.; Baker, M. A.; Oguma, T.; Burdge, H. E.; Kawajiri, T.; Shenvi, R. A. Concise syntheses of GB22, GB13 and himgaline by cross-coupling and complete reduction. Science, 2022, 375, 1270–1274.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
64. Tong, G.; Shenvi, R. A. Revision of the unstable picrotoxinin hydrolysis product Angew. Chem. Int. Ed. 2021, 60, 19113.
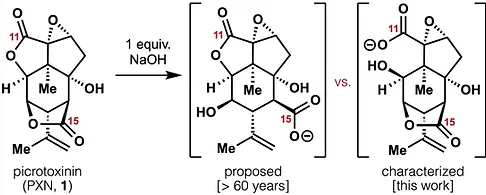
62. Woo, S.; Shenvi, R. A. Natural Product Synthesis through the Lens of Informatics Acc. Chem. Res. 2021, 54, 1157–1167.
61. Tong, G.; Baker, M. A.; Shenvi, R. A. Change the channel: CysLoop receptor antagonists from Nature. Pest Manag. Sci. 2020, 77, 3650–3662. [Special Issue honoring Tom Sparks for the Kenneth Spencer Award]
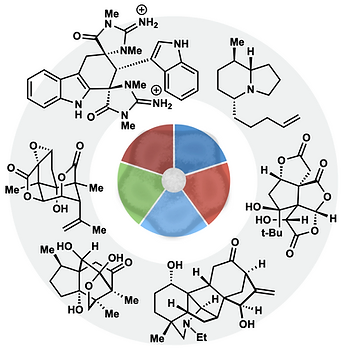
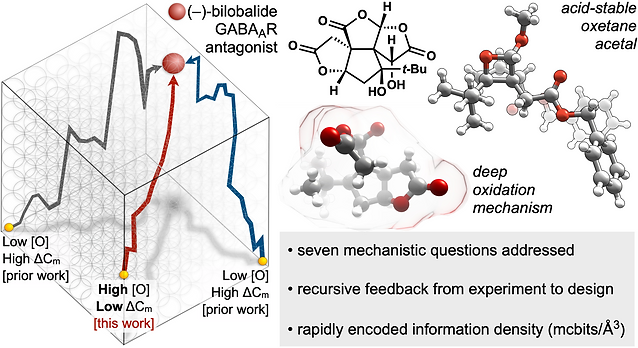
59. Demoret, R. M.; Baker, M. A.; Ohtawa, M.; Chen, S.; Lam, C.-C.; Khom, S.; Roberto, M.; Forli, S.; Houk, K.; Shenvi, R. A. Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide. J. Am. Chem. Soc. 2020, 142, 18599–18618.ChemRxiv DOI: 10.26434/ chemrxiv.12132939.v2
58. Hill, S. J.; Brion, A. U. C. M.; Shenvi, R. A. Chemical Syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020, 37, 1478–1496.

57. Crossley, S. W. M.; Tong, G.; Lambrecht, M. J.; Burdge, H. E.; Shenvi, R. A. Total Synthesis of (–)-Picrotoxinin J. Am. Chem. Soc. 2020, 142, 11376–11381.
ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1
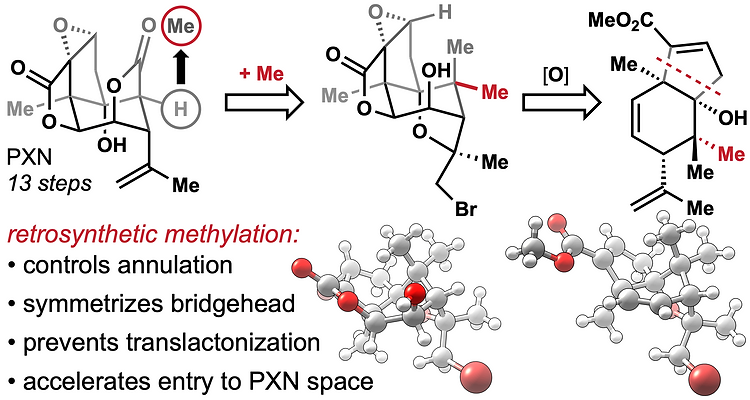
55. Burdge, H. E.; Oguma, T.; Kawajiri, T.; Shenvi, R. A. Concise Synthesis of GB22 by Endo-Selective Siloxycyclopropane Arylation ChemRxiv DOI: 10.26434/chemrxiv.8263415.v1 .
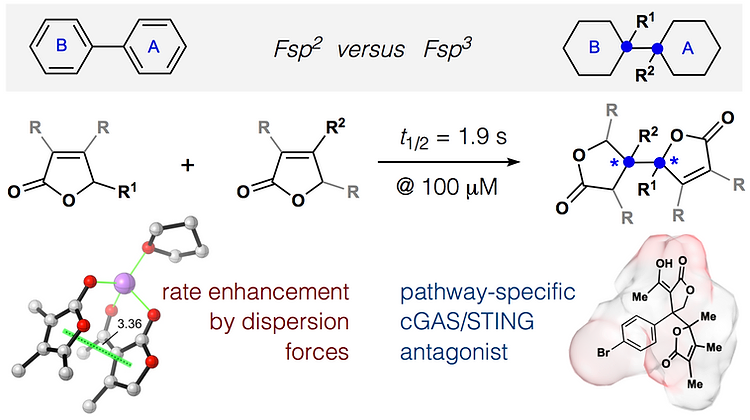
54. Huffman, B. J.; Chen, S.; Schwarz, J. L; Plata, R. E.; Chin, E.; Houk, K. N.; Lairson, L. L.; Shenvi, R. A. Electronic Complementarity Permits Hindered Butenolide Heterodimerization and Discovery of Novel cGAS/STING Pathway Antagonists, Nature Chem. 2020, 12, 310–317.
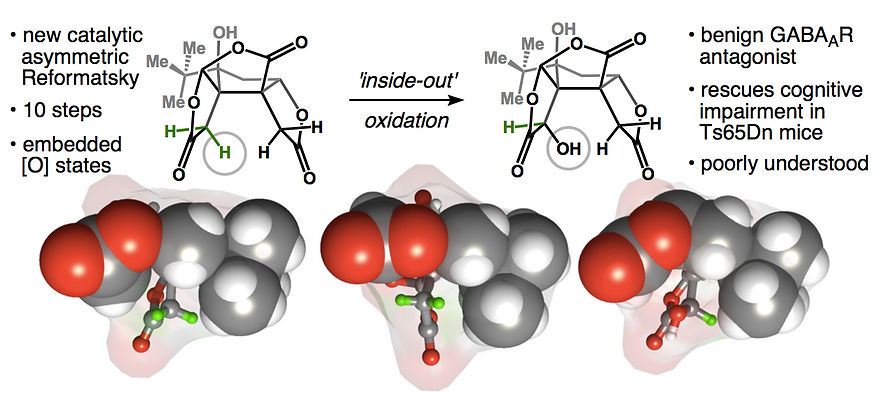
51. Baker, M.; Demoret, R.; Ohtawa, M.; Shenvi, R. A. Concise Asymmetric Synthesis of (–)-Bilobalide Nature 2019, 575, 643–646. DOI: 10.1038/s41586-019-1690-5. ChemRxiv DOI: 10.26434/chemrxiv.8202053.v1 Featured in Org. Chem. Highlights
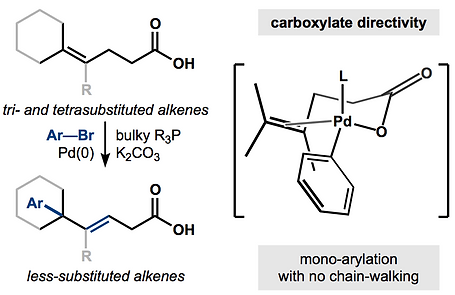
47. Huffman, T.; Wu, Y.; Emmerich, A.; Shenvi, R.A. Intermolecular Heck Coupling with Hindered Alkenes Directed by Potassium Carboxylates Angew. Chem. Int. Ed. 2019, 58, 2371-2376.
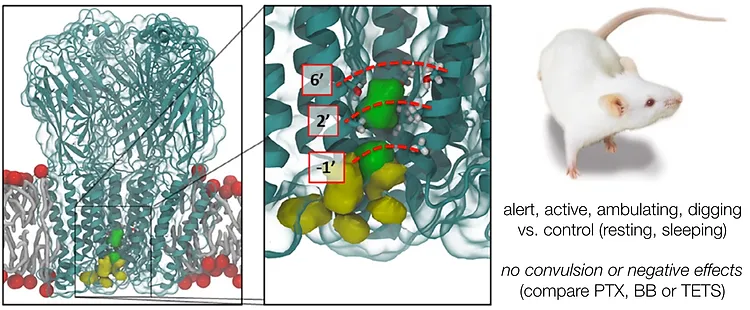
42. Jeffrey M. Witkin, Ryan A. Shenvi, Xia Li, Scott D. Gleason, Julie Weiss, Denise Morrow, John T. Catow, Mark Wakulchik, Masaki Ohtawa, Hai-Hua Lu, Michael D. Martinez, Jeffrey M. Schkeryantz, Timothy S. Carpenter, Felice C. Lightstone, Rok Cerne Pharmacological characterization of the neurotrophic sesquiterpene jiadifenolide reveals a non-convulsant signature and potential for progression in neurodegenerative disease studies. Biochem. Pharm. 2018, 155, 61–70.
DOI: 10.1016/j.bcp.2018.06.022
40. Roach, J. J.; Shenvi, R. A. A Review of Salvinorin Analogs and their Kappa-Opioid Receptor Activity. Bioorg. Med. Chem. Lett. 2018, 28, 1436–1445. DOI: 10.1016/j.bmcl.2018.03.029
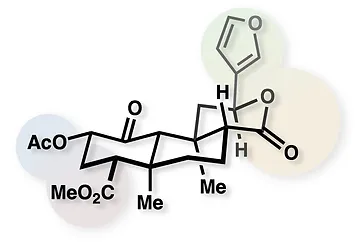
39. Hirasawa, S.; Cho, M.; Brust, T. F.; Roach, J. J.; Bohn, L. M.; Shenvi, R. A. O6C-20-nor-SalA is a stable and potent KOR agonist. Bioorg. Med. Chem. Lett. 2018, 28, 2770–2722. Special Issue dedicated to Dale Boger.
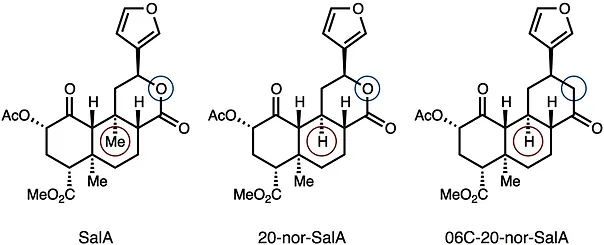
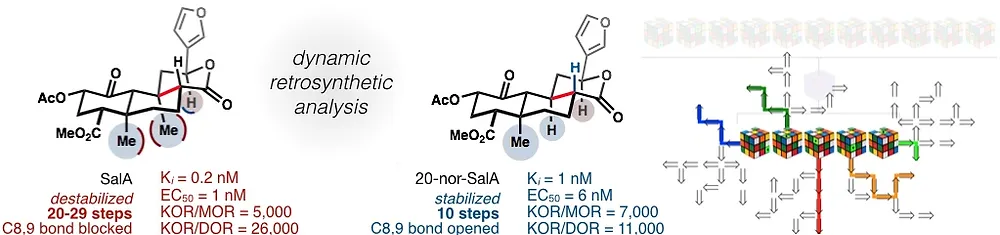
38. Dynamic Strategic Bond Analysis Yields a 10-step Synthesis of 20-nor-SalA, a Potent Κ-OR Agonist
38. Roach, J. J.; Sasano, Y.; Schmid, C. L.; Zaidi, S.; Katrich, V.; Stevens, R. C.; Bohn, L. M.; Shenvi, R. A. Dynamic Strategic Bond Analysis Yields a 10-step Synthesis of 20-nor-SalA, a Potent Κ-OR Agonist. A(CS)2 2017, 3, 1329–1336.
ChemRxiv DOI: 10.26434/chemrxiv.5318188
- #1 Most Viewed Article on ChemRxiv, Aug-Dec 2017.
- Highlighted by Tien Ngyuen in C&EN, Sept. 1 2017.
- See article on SlashGear by Brittany Rosen, Sept. 11, 2017
- See article on Gizmodo by Ryan Mandalbaum, Sept. 12, 2017
- See article on Geek by Daniel Starkey, Sept. 12, 2017
- See article on Wired by Matthew Simon, Jan. 8, 2018
37. Ohtawa, M.; Krambis, M. J.; Cerne, R.; Schkeryantz, J.; Witkin, J. M.; Shenvi, R. A. Synthesis of (–)-11-O-Debenzoyltashironin: Neurotrophic Sesquiterpenes Cause Hyperexcitation. J. Am. Chem. Soc. 2017, 139, 9637-9644.
Featured in Org. Chem. Highlights
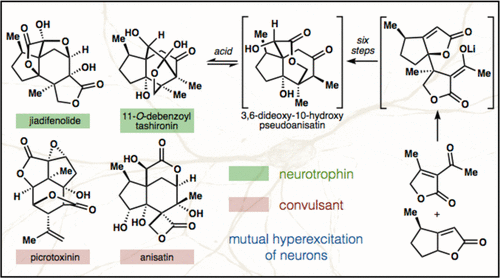
28. Shenvi, R. A. Neurite Outgrowth Enhancement by Jiadifenolide: Possible Targets. Nat. Prod. Rep. 2016, 33, 535-539.
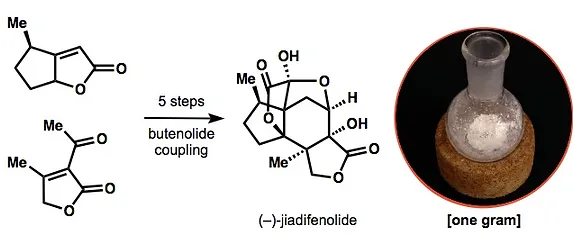
24. Lu, H.-H.; Martinez, M. D.; Shenvi, R. A. Eight-Step, Gram-Scale Synthesis of (–)-Jiadifenolide. Nature Chem. 2015, 7, 604-607.
13. Pronin, S. V.; Tabor, M. G.; Jansen, D. J.; Shenvi, R. A. A Stereoselective Hydroamination Transform to Access Polysubstituted Indolizidines. J. Am. Chem. Soc. 2012, 134, 2012-2015.
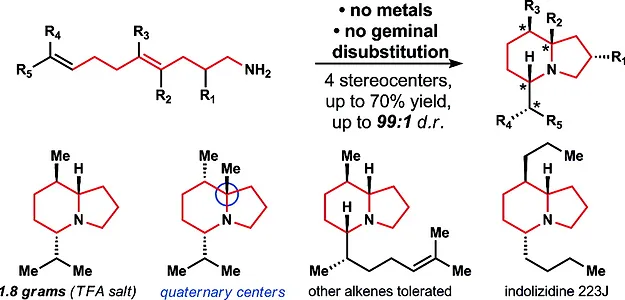
- In top ten most accessed articles of J. Am. Chem. Soc. for January 2012.
- Highlighted in Nature Chemistry, May 2012.